Inferior Vena Cava Thrombosis Secondary to Liver Hydatid Cyst: Case Report
Naser A;
* Alqader RS;
Ghanem L;
Shareef ZA;
Al-asbahi HM;
Abukheat N;
-
Naser A: Department of Medicine, Al Bashir Hospital, Amman, Jordon.
-
* Alqader RS: Department of Medicine, University of Jordan, Amman, Jordan.
-
Ghanem L: Department of Medicine, University of Jordan, Amman, Jordan.
-
Shareef ZA: Department of Medicine, University of Jordan, Amman, Jordan.
-
Al-asbahi HM: Department of Medicine, Al Bashir Hospital, Amman, Jordon.
-
Abukheat N: Department of Medicine, University of Jordan, Amman, Jordan.
-
Sep 18, 2024 |
-
Volume: 5 |
-
Issue: 1 |
-
Views: 1540 |
-
Downloads: 1207 |
Abstract
Hydatidosis is a zoonotic disease that can affect various parts of the body, primarily the liver, leading to the development of parasitic cystic lesions following the ingestion of food contaminated with animal feces. The disease’s prevalence in Jordan varies based on residency location, educational status, and proximity to herbivorous animals and dogs, which are key hosts. This case study presents a 45-year-old Syrian male patient living in the Al-Zaatary refugee camp in Jordan who works as a blacksmith. He initially sought medical care due to recurrent right-sided loin pain despite normal lab results. Imaging revealed a cystic mass in the seventh segment of the liver, complicated by inferior vena cava thrombosis. This case emphasizes the importance of early detection and accurate diagnosis of hydatid cysts, particularly those complicated by inferior vena cava thrombosis.
Introduction
Hydatidosis is a zoonotic disease that could potentially affect different parts of the body, primarily the liver (around 75% of cases) and, less commonly, the lungs [1]. Cestode species within the genus Echinococcus are responsible for various types of infections. Echinococcus granulosus causes Cystic Echinococcosis (CE), while E. multilocularis leads to Alveolar Echinococcosis (AE). On the other hand, polycystic forms result from infections caused by either E. vogeli or E. oligarthrus. The tapeworm Echinococcus granulosus remains the most common, and its larval forms result in growing parasitic cystic lesions following the ingestion of food contaminated with animal feces [2].
The distribution of Echinococcus varies globally; in the Middle East, it is considered a rising issue and is a major health problem. To specify, Jordan and its neighboring countries are considered endemic for cystic Echinococcosis, with research showing a seroprevalence of 3.5% in Jordan’s general population and 7.7% in individuals at risk, which are significant percentages [3,4]. Understandably, there is an apparent difference in the disease prevalence between different people within the country, based on the location of residency, educational status, and living with herbivore animals, including sheep, goats, cattle, and most importantly, close contact with dogs which are the main definitive hosts of the disease in Jordan, with research recovering parasites from up to 14% of examined stray dogs [5,6].
The patient with this disease is usually asymptomatic for a long duration due to the slow growth of the cyst. However, the presenting symptoms depend on the size, number, mass effect, and complications of the cyst. Live hydatidosis may present with hepatomegaly, right-sided/epigastric pain, nausea, and vomiting [7].
Hydatid cyst lesions could lead to an array of relatively common critical complications, mainly including super-infections, rupture into the neighboring structures such as the biliary tree or the abdominal cavity, and compression of adjacent vessels due to its high pressure [8–10]. It is important to note that the recognition of any disease is a very important aspect of determining the appropriate surgical management while achieving the least possible amount of morbidity and mortality.
The silent nature of the illness means that the diagnosis of a liver hydatid cyst is often incidental to abdominal ultrasonography performed due to other causes. A combination of imaging, serologic, and immunologic studies is needed for a definitive diagnosis of liver echinococcosis to be made. Ultrasonography (US) is considered the screening method of choice. CT scan is an essential preoperative diagnostic tool for recognition of cyst extension into the vascular system, biliary tree, or extrahepatic extension, and complications such as cyst rupture and infections [11].
Imaging features of hydatid cysts are variable and may be confused with other lesions, such as simple cysts early on in the disease course and different types of liver malignancies later on. Thus, narrowing down a list of differentials of cystic space-occupying lesions in the liver and reaching a spot-on diagnosis is challenging and may require the assessment of minor details such as the pressure of the cyst, which is higher in parasitic cysts than simple cysts, with a value of 30 mmHg–80 mmHg [12–14].
That said, those working in endemic areas should have a high index of suspicion to avoid a late or incorrect diagnosis and consequent complications. This is demonstrated in this case report, which emphasizes the importance of early detection and accurate diagnosis of Hydatid cysts.
Case Presentation
A 45-year-old Syrian male patient from the Al-Zaatary refugee camp in Jordan, who works as a blacksmith in various locations, including sheep and cattle farms, initially sought medical attention in March 2023 from the private sector due to recurrent attacks of right-sided loin pain. The patient reported radiation of the pain to the right shoulder, accompanied by fatigue, nausea, and dizziness. There was no weight loss, no abnormal change in bowel habits, or any other related symptom. His medical and family history were unremarkable.
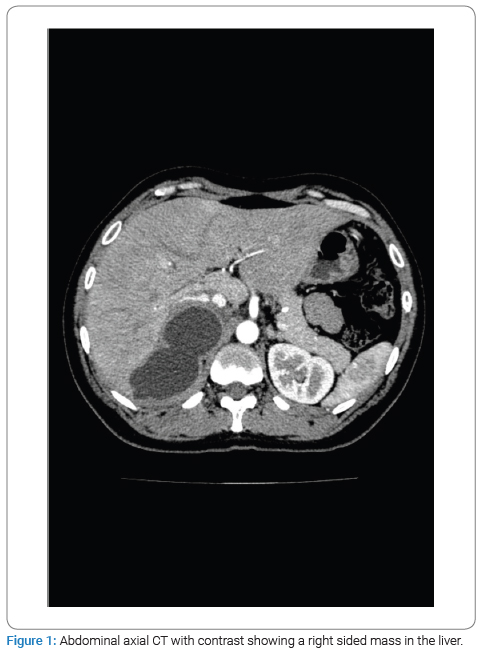
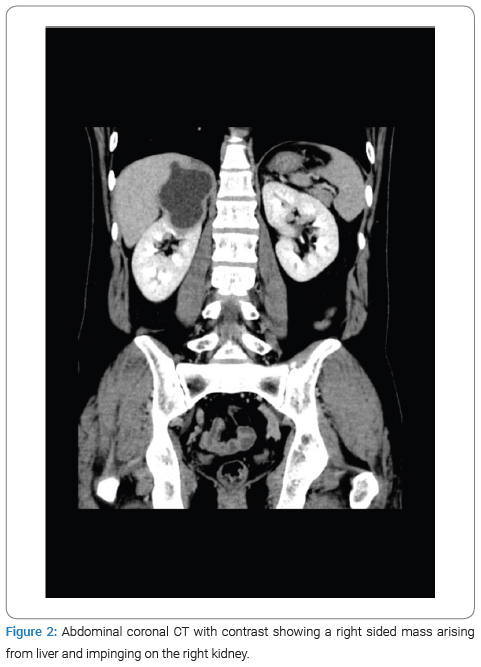
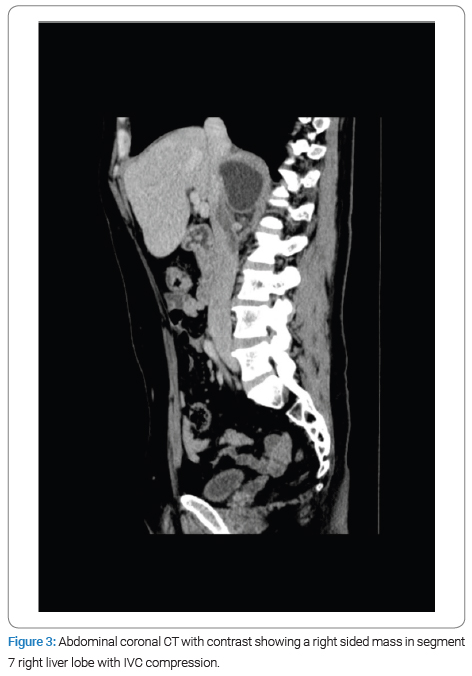
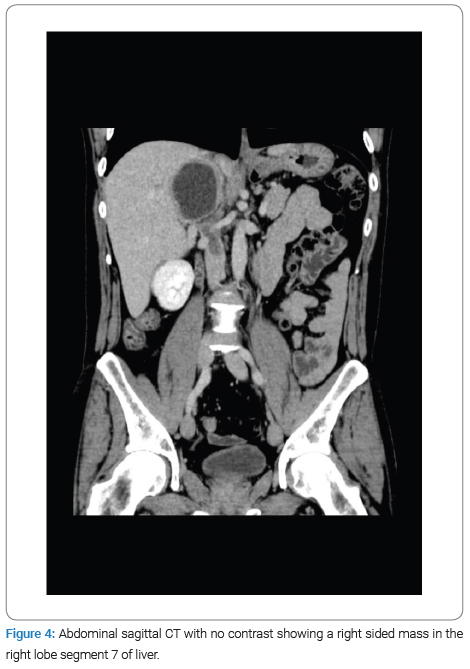
The physical examination revealed no jaundice and a soft, lax abdomen with right upper quadrant tenderness, as well as bilateral pitting lower limb edema, which was associated with numbness and bluish discoloration of the foot. Consequently, imaging studies were done for the patient, among which a CT scan with IV contrast identified a right-sided abdominal cystic lesion, leading to suspicions of either an adrenal or renal mass. Subsequently, the patient was transferred to Al-Bashir Hospital, where additional investigations were conducted. A triphasic CT scan revealed a cystic mass located in the seventh segment of the liver, measuring 10.4 cm × 5.5 cm × 7.6 cm, with a non-enhancing thick wall and a fluid density (Figure 1). As a result of the cyst, impingement of the right kidney was observed (Figure 2), along with compression of the suprarenal and infra-hepatic portion of the inferior vena cava with associated thrombosis, as shown in (Figure 3). Lab results revealed leukocytes: 7.15 × 10^3 /UL, Hb: 13.4 g/dL, eosinophils: 1.4% (0.9–6), AST: 25.1, ALT: 37.2, total bilirubin: 5.1 umol/L (5.1–17.1), and Cr: 50 mol/L. Positive serological testing confirmed the presence of a hydatid cyst (Figure 4).
After careful clinical and radiologic evaluation of the patient’s data, the patient has prescribed a therapeutic dose of low molecular weight heparin and 15 mg/kg/day Albendazole for one-month duration to manage the IVC thrombosis, followed by surgical laparoscopic de-roofing of the cyst using 1% Cetramide. Intraoperatively, a cyst in the seventh segment of the liver was found, compressing the IVC. After isolating the surroundings of the cyst with Cetramide-soaked gauze, decompression of the cyst was performed via aspiration of its fluid content. Then, Cetramide was injected into the cyst, and de-roofing was done ten minutes later, removing the laminated membrane. The crystal-clear color of the fluid and the laminated membrane were consistent with the hydatid cyst. Simultaneously, a biopsy was taken and sent to histopathology for examination, in which multiple whitish membranes and dark gray soft tissue fragments measuring in aggregates 1.5 cm × 1.5 cm were seen. The pathology results confirmed the diagnosis.
The patient’s recovery following the operation was smooth, without any complications or evidence of thrombus showering. He was discharged on the second postoperative day and continued on Albendazole for three additional months. He remains in a healthy condition without recurrence to this date.
Discussion
Hydatid disease is a parasitic infection in humans caused by the larval forms of E. granulosus that results in growing parasitic cystic lesions [2]. A hydatid cyst is a fluid-filled vesicle consisting of an internal germinal layer followed by a laminated layer and an outer layer consisting of fibrotic tissue [15,16].
Ultrasound is one of the imaging modalities used to evaluate the size, position, and morphology of cysts, according to the standardized ultrasound classification suggested by the World Health Organization. Hydatid cysts have five distinctive categories based on their morphology and structure. CE1 and CE2 represent active cysts, Whereas type CE3 is a transitional, degenerating cyst. On the other hand, types CE4 and CE5 suggest inactive cysts [8]. In our case, the cyst was classified as CE1/CE2, which was among the reasons that led to the initial misdiagnosis with the adrenal cyst.
The diagnosis of hepatic hydatid cysts can be tricky, mainly due to the fact that they may resemble simple cysts, especially in the early stages [6]. Tests such as latex agglutination, counter immunoelectrophoresis, Enzyme-Linked Immunosorbent Assay (ELISA), and other tests have been shown to have lesser sensitivity and specificity when compared to imaging modalities [17,18]. Furthermore, Only 39% of patients present with eosinophilic elevation [19]; therefore, a single serological test cannot give a definitive diagnosis. The clinical symptoms of a hydatid cyst, such as the ones mentioned above, depend on the size and anatomical position of the cyst in relation to its adjacent structures and associated complications, such as secondary infection. In our case, there were no specific signs or symptoms that favored a specific diagnosis, even though images showed the hepatic hydatid cyst compressing the IVC. Moreover, the location of the cyst was in close proximity to the adrenal glands, and a cystic impingement was noticed on the right kidney. As a result, the cyst was initially mistaken for a renal/adrenal cyst; this complex location of the cyst created diagnostic difficulties for clinicians and widened the suspected possible differential diagnosis.
In this case, the diagnosis of a hydatid cyst was eventually determined by considering multiple methods, such as serological tests, imaging, and the patient’s geographical location. The fluid inside a hydatid cyst exerts pressure on its walls. The usual intracystic pressure (ICP) falls within the 30 mmHg–80 mmHg range. Unlike non-parasitic hepatic cysts exhibiting lower ICP, this increased pressure has been associated with a heightened risk of complications [14]. In our study, the higher intracystic pressure supported a leaning towards diagnosing a hydatid cyst rather than other cysts because this increased pressure is more commonly associated with Echinococcus cysts.
Diagnostic difficulties were pointed out in multiple studies presenting the challenge of differentiating hepatic hydatid cysts from simple cysts and other cysts where images or serological tests alone aren’t sufficient to make a diagnosis. However, a combination of imaging examinations and serological testing can provide a more reliable diagnosis [20,21]. This explains why our case was misdiagnosed initially; a similar Case report pointed out the confusion between a hepatic hydatid cyst and a simple cyst where the diagnosis wasn’t straightforward due to the absence of symptoms and the complex anatomical position and size of the cyst [12].
Only a few case reports about hydatid cysts compressing the IVC are found in the literature. Rajagopal et al. [22] Reported a case with a Hepatic hydatid cyst compressing the IVC; however, the patient showed obvious signs of IVC obstruction, unlike our case, where the signs were not very specific. Other reports discussed the finding of a hepatic hydatid cyst compressing the IVC for a patient who was admitted for renal disease. And a case of Hydatidosis with IVC obstruction that was discovered on an autopsy [23,24].In our case, the pressure exerted by the hydatid cyst on the IVC led to thrombus formation; this aligns with several papers in the literature that reported Thrombosis of the Retrohepatic Vena Cava caused by a Hepatic Hydatid Cyst as well as a situation when a patient’s inferior vena cava, left renal, right common iliac, and right external iliac veins all were thrombosed as a result of an infected hepatic hydatid cyst that ruptured spontaneously into the IVC. Furthermore, there was a more severe case where a patient experienced Pulmonary Embolism due to hepatic hydatid cyst rupture leading to IVC thrombosis [25–27].
Hydatid cysts of the liver can be treated in various ways, surgically, by percutaneous drainage, or with medication [28]. In our case, the cyst was managed with Laparoscopic Surgery. Multiple things need to be taken into consideration when choosing the appropriate treatment; in the literature, Similar cases to ours were treated differently; for instance, in Rajagopal et al. [19], Due to the patient’s low albumin level and malnourished status, surgical intervention was not considered. Instead, paracentesis was performed, whereas, in a second report, the first step was surgical exploration followed by surgical excision. This proves the necessity to individually customize treatment to each of the many clinical scenarios; the combined medicinal, interventional radiology and surgical therapies may represent the gold standard in the management of large, complicated hepatic hydatid cysts [29].
Conclusion
To conclude, the following case report demonstrates the difficulties faced in diagnosing hydatid cysts, specifically in the liver. Multiple factors were misleading when our patient was being diagnosed, including the presence of CE1/CE2, as it is usually a feature of a simple cyst. The absence of specific signs and symptoms, the challenging location of the cyst, as well as, the low sensitivity of eosinophilic elevation in the blood tests contributed to the difficulty of diagnosis. Hydatid cyst lesions have the potential to cause various common and serious complications. These complications primarily involve super-infections, the cyst rupturing into nearby structures like the biliary tree or abdominal cavity, and putting pressure on surrounding blood vessels.
This case report emphasizes the importance of adding hydatid cysts to the list of differential diagnoses of cystic lesions, especially in patients with a high-risk history, such as those residing in endemic areas or exposure to contaminated food. Physicians should use a combination of imaging studies, serology, and clinical presentation to reach high clinical suspicion. Early and correct diagnosis is a key element in treating patients successfully.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid disease from head to toe. Radiographics. 2003;23(2):475–494; quiz 536–537.
- Bourée P. Hydatidosis: dynamics of transmission. World J Surg. 2001;25(1):4–9.
- Sadjjadi SM. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol Int. 2006:55 Suppl:S197–S202.
- Galeh TM, Spotin A, Mahami-Oskouei M, Carmena D, Rahimi MT, Barac A, et al. The seroprevalence rate and population genetic structure of human cystic echinococcosis in the Middle East: A systematic review and meta-analysis. Int J Surg. 2018:51:39–48.
- el-Shehabi FS, Kamhawi SA, Schantz PM, Craig PS, Abdel-Hafez SK. Diagnosis of canine echinococcosis: comparison of coproantigen detection with necropsy in stray dogs and red foxes from northern Jordan. Parasite. 2000;7(2):83–90.
- Ajlouni AQ, Saliba EK, Disi AM. Intestinal cestodes of stray dogs in Jordan. Z Parasitenkd. 1984;70(2):203–210.
- Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, et al. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18(13):1448–1458.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114(1):1–16.
- Symeonidis N, Pavlidis T, Baltatzis M, Ballas K, Psarras K, Marakis G, et al. Complicated liver echinococcosis: 30 years of experience from an endemic area. Scand J Surg. 2013;102(3):171–177.
- Chautems R, Bühler LH, Gold B, Giostra E, Poletti P, Chilcott M, et al. Surgical management and long-term outcome of complicated liver hydatid cysts caused by Echinococcus granulosus. Surgery. 2005;137(3):312–316.
- Derbel F, Mabrouk MB, Hamida MBH, Mazhoud J, Youssef S, Ali AB, et al. Hydatid cysts of the liver-diagnosis, complications and treatment. Abdominal Surgery. 2012;5:105–138.
- Xu Y, Hu X, Li J, Dong R. Hepatic Hydatid Cyst Misdiagnosed as Simple Cyst: A Case Report. Am J Case Rep. 2020;21:e923281.
- Chouhan MD, Wiley E, Chiodini PL, Amin Z. Hepatic alveolar hydatid disease (Echinococcus multilocularis), a mimic of liver malignancy: a review for the radiologist in non-endemic areas. Clin Radiol. 2019;74(4):247–256.
- Yalin R, Aktan AO, Yeğen C, Döşlüoğlu HH. Significance of intracystic pressure in abdominal hydatid disease. Br J Surg. 1992;79(11):1182–1183.
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17(1):107–135.
- Caremani M, Lapini L, Caremani D, Occhini U. Sonographic diagnosis of hydatidosis: the sign of the cyst wall. Eur J Ultrasound. 2003;16(3):217–223.
- Babba H, Messedi A, Masmoudi S, Zribi M, Grillot R, Ambriose-Thomas P, et al. Diagnosis of human hydatidosis: comparison between imagery and six serologic techniques. Am J Trop Med Hyg. 1994;50(1):64–68.
- Sbihi Y, Rmiqui A, Rodriguez-Cabezas MN, Orduña A, Rodriguez-Torres A, Osuna A. Comparative sensitivity of six serological tests and diagnostic value of ELISA using purified antigen in hydatidosis. J Clin Lab Anal. 2001;15(1):14–18.
- Botezatu C, Mastalier B, Patrascu T. Hepatic hydatid cyst - diagnose and treatment algorithm. J Med Life. 2018;11(3):203–209.
- Oruç E, Yıldırım N, Topal NB, Kılıçturgay S, Akgöz S, Savcı G. The role of diffusion-weighted MRI in the classification of liver hydatid cysts and differentiation of simple cysts and abscesses from hydatid cysts. Diagn Interv Radiol. 2010;16(4):279–287.
- Ran B, Aji T, Jiang T, Zhang R, Guo Q, Abulizi A, et al. Differentiation between hepatic cystic echinococcosis types 1 and simple hepatic cysts: A retrospective analysis. Medicine (Baltimore). 2019;98(1):e13731.
- Rajagopal KV, Bishwas R. Hydatid cyst of the liver presenting as an inferior vena cava obstruction. J Clin Ultrasound. 2002;30(2):114–116.
- Kilciler M, Bedir S, Erdemir F, Coban H, Sahan B, Ozgok Y. Isolated unilocular renal hydatid cyst: a rare diagnostic difficulty with simple cyst. Urol Int. 2006;77(4):371–374.
- Aikat BK, Bhusnurmath SR, Cadersa M, Chhuttani PN, Mitra SK. Echinococcus multilocoularis infection in India: First case report proved at autopsy. Trans R Soc Trop Med Hyg. 1978;72(6):619–621.
- Nagarajan K, Sekar D, Babu JV, Kamath A. Hydatid cyst of the liver causing inferior vena caval obstruction. J Assoc Physicians India. 2013;61(9):671–673.
- Gruttadauria S, Luca A, Cintorino D, Doria C, Scott VL, Marino IR. Hepatic hydatid cyst causing thrombosis of the inferior vena cava and complicated by hemobilia: a multimodal sequential approach in the treatment. Dig Dis Sci. 2003;48(2):358–364.
- Anuradha S, Agarwal SK, Khatri S, Bhasin S, Singh NP, Chowdhury V. Spontaneous rupture of hepatic hydatid cyst causing inferior vena cava thrombosis. Indian J Gastroenterol. 1999;18(1):34.
- Poyraz N, Demirbaş S, Korkmaz C, Uzun K. Pulmonary embolism originating from a hepatic hydatid cyst ruptured into the inferior vena cava: CT and MRI Findings. Case Rep Radiol. 2016;2016:3589812.
- Dziri C, Haouet K, Fingerhut A. Treatment of hydatid cyst of the liver: where is the evidence? World J Surg. 2004;28(8):731–736.
Keywords
Hydatid cyst; Inferior vena cava thrombosis; Vascular compression; Echinococcus granulosus
Cite this article
Naser A, Alqader RS, Ghanem L, Shareef ZA, Al-asbahi HM, Abukheat N. Inferior vena cava thrombosis secondary to liver hydatid cyst: case report. Clin Surg J. 2024;5(1):1–5.
Copyright
© 2024 Alqader RS. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




