Epidemio-Clinical Profile and Treatment of Aneurysms on Native Arteriovenous Fistula Waiting for Chronic Hemodialysis in Ivory Coast
* Ayegnon KG;
Diby KF;
Abro S;
Choho MCC;
Gnaba LA;
Amani KHA;
Ouattara PE;
Diomandé M;
Binaté A;
Adoubi KA;
-
* Ayegnon KG: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Diby KF: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Abro S: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Choho MCC: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Gnaba LA: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Amani KHA: Department of Surgery, Felix Houphouët-Boigny University, Cocody-Abidjan, Côte d’Ivoire.
-
Ouattara PE: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Diomandé M: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Binaté A: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Adoubi KA: Department of Surgery, Alassane Ouattara University of Bouaké, Bouaké, Côte d’Ivoire.
-
Jan 16, 2023 |
-
Volume: 4 |
-
Issue: 1 |
-
Views: 2335 |
-
Downloads: 1617 |
Abstract
This study identifies fistulized renal patients likely to develop this type of postoperative complication in order to improve their treatment.
Material and methods: This was a cross-sectional study of patients with arteriovenous fistula who were received for Arteriovenous Aneurysm (AVA) in the main Emergency Medical Care and Assistance Services (SAMU) of Abidjan (University Hospital of Cocody, Yopougon, and Treichville) between March 2014 and February 2018 and of Bouaké from March 2018 to September 2021. We recorded epidemiological, clinical, and biological data for each AVA carrier, HIV seroprevalence, surgical procedures, and postoperative.
Results: 2013 NAVF were collected with 117 AAV (5.9%) during this period. The mean age was 42.30 years ± 13.31 years [extremes: 11 years and 75 years], and the sex ratio (M/F) was 2.36. The age range of 10 years to 50 years represented 68.38%. 52.14% of patients developing AVA were unemployed or were working in the informal sector. HIV seroprevalence was 11.97%. The main cardiovascular risk factor was hypertension (84.11%). The mean pre-hospital renal clearance was 39.7 ml/min ± 1.2 ml/min [extremes: 17 ml/min and 91 ml/min]. NAVF was located mainly in the left forearm (86.09%). AVAs were located at the site of the NAVF (69.23%) and at the site of puncture of the arterialized vein (30.77%), with a mean time of occurrence of 18.14 months ± 12 months. False aneurysms and true AVAs, respectively, represented 17.09% and 82.91%. Fifty patients underwent reoperation for an AVA. Surgery consisted of total aneurysmectomy (n = 41), initial removal of the NAVF followed by immediate remote NAVF reconfection (n = 10), and secondary (n = 31). The mean duration of hospitalization was 6.13 ± 4.49 hours [extremes: 0.5 and 78 hours]. The loss of sight rate was 4.5%. Immediate postoperative complications of AVA (34%) were due to fistulous hyper flow syndrome (n = 7), surgical wound suppuration (n = 4), AVA recurrence (n = 2), severe anemia, and hemorrhage (n = 4). Immediate postoperative mortality was 18% versus 10.30% with surgical abstention (p = 0.48).
According to the pathological anatomical aspect of the arteriovenous lesion in our study, the predictor of reoperation was HIV seropositivity adjusting for false arteriovenous aneurysms HRa = 3.098; CI95 [1.3217–7.2647], p = 0.0008.
Introduction
The American Society of Vascular Surgery defines a true aneurysm as a circumscribed dilation of all three vascular layers in contrast to a pseudoaneurysm, representing a focal dilation of the vascular wall by neointimal and fibrous tissue that can involve both artery and vein [1]. Autologous or Native Arteriovenous Fistula (NAVF) may be complicated by a true Arteriovenous Aneurysm (AVA) or a pseudoaneurysm-like formation [1,2]. In the current K/DOQI guidelines, an aneurysm is defined as an abnormal blood-filled dilation of the blood vessel wall resulting from vessel wall disease, and a pseudoaneurysm is a vascular anomaly that resembles an aneurysm, but the expansion is not bounded by a true vessel wall, but rather by external fibrous tissue [3]. Thus, arteriovenous aneurysms can be true or false [2,3]. They are either congenital or acquired most often. When these AVAs are acquired, vascular trauma is their main etiology [4]. In the West, arteriovenous surgery, particularly the creation of native arteriovenous fistulas, results in 4% to 12% of AVAs [5]. In Côte d’Ivoire, where the NAVF has been undergoing a revival of interest since the 2000s because of the multiplication of cases during end-stage renal disease, no epidemiological and surgical data are available on AVA following NAVF. Therefore, we undertake this study to specify the epidemiological and clinical profile and the surgical risk factors in order to improve the surgical management of AVA following NAVF while waiting for hemodialysis through the identification of renal insufficiency patients likely to develop this post-NAVF AVA.
Material and Methods
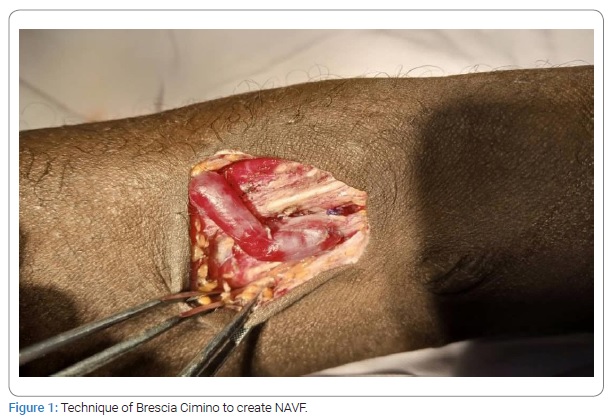
Between March 2014 and February 2018 in Abidjan and from March 2018 to September 2021 in Bouaké, we performed a cross-sectional study of patients received in the main Emergency Medicine Care and Assistance Services (SAMU) of Abidjan (University Hospital of Cocody, Yopougon, and Treichville) and of Bouaké. The surgical technique of Brescia Cimino [6] was used in the preparation of all the NAVF (Figure 1). In case of occurrence of post-NAVF AVA whose clinical size was greater than 20 mm, the criteria for the operability of these AVA were: Pain, ultrasound external diameter > 35 mm, and AVA rupture. Beyond these criteria, we studied HIV seroprevalence, epidemiological, clinical, biological, Doppler ultrasound characteristics, surgical indications, postoperative results, follow-up, and risk factors of AVA reinterventions. Arteriovenous Doppler echocardiography was used when there was evidence of clinical deterioration caused in the end-stage renal disease patient by injury to the native arteriovenous fistula (stenosis, thrombosis, deterioration, or insufficiency) or its pathway. Follow-up data and information were complete in 96.5% of patients. Clinical assessments were obtained during inpatient visits, telephone interviews, or letters addressed directly to the attending nephrologist or to the emergency physicians of the SAMU (Emergency Medical Care and Assistance Services). In case of a lack of reply, the contact of other resource persons (parents, municipal authorities) was necessary to complete our cross-sectional study at the peak date. The terms morbidity, mortality, 30-day mortality, and in-hospital mortality were used according to the definitions of the “Guidelines” after vascular surgery procedures [7].
The prevalence of arteriovenous aneurysms on native arteriovenous fistulas being of the order of 4% in the literature, the expected sample by applying the statistical formula is equal to (1.962 x (0.04) (1–0.04)/0.052), i.e., 59 patients with a precision of 0.05%. Given the existence of two cardiovascular surgery centers in the Ivory Coast, and the underestimation of the parent population of insufficient renal patients who receive regular hemodialysis, the inclusion rate was doubled. Thus, for a representative sample, we successively included all patients with arteriovenous aneurysms consecutive to a native arteriovenous fistula that met the research protocol, i.e. (59 x 2-1). All patients with a Native Arteriovenous Fistula (NAVF) usually followed in nephrology, and Emergency Medical Care and Assistance services and who present a deformation or alteration of the fistula site or path, thrombosis, pre-rupture syndrome or rupture of the NAVF are referred to a cardiovascular surgery service.
Clinical, biological, ultrasonographic, diagnostic, operative data, and follow-up medical record is written and kept.
A complete medical record is summarized on a protocol reading sheet targeting the variables in our study. Both in and out of the hospital, postoperative adverse events (complications, deaths, their causes, and dates of occurrence) were recorded by the medical visit to the bed, telephone, or postal survey taking into account their dates when they occurred. The research protocol reading sheets identified missing data on the arteriovenous aneurysm database following NAVF. Quantitative variables were expressed as mean ± Standard Deviations (SD). Comparisons were made using the Chi-square, Fisher, and Mantel Haenszel tests for qualitative variables. For all these tests, the significance level was p < 0.05.
Exclusion criteria: All patients carrying NAVF who developed AVA outside the upper extremity venous network used for puncture for chronic hemodialysis were excluded from the study.
Patients who did not respond to the follow-up protocol or telephone/mail surveys and had missing data greater than 10% of the protocol items were excluded after one month of attempts to harmonize the database by the cardiovascular surgery resident.
Definitions:
- The pre-hospital renal clearance is calculated during the preoperative check-up of the native arteriovenous fistula.
- Immediate mortality is all-cause mortality at 30 days, regardless of location (home or health care facility).
- The terms morbidity, mortality, 30-day mortality, and in-hospital mortality were used according to the definitions of the “Guidelines” after vascular surgery procedures [7].
Results
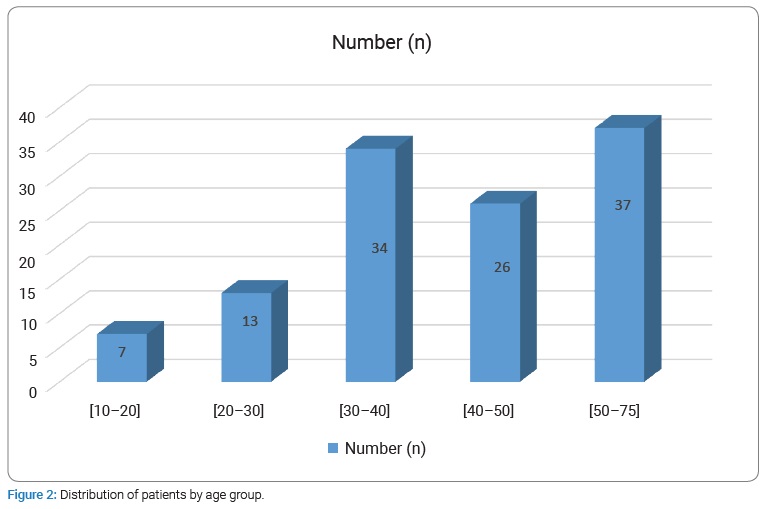
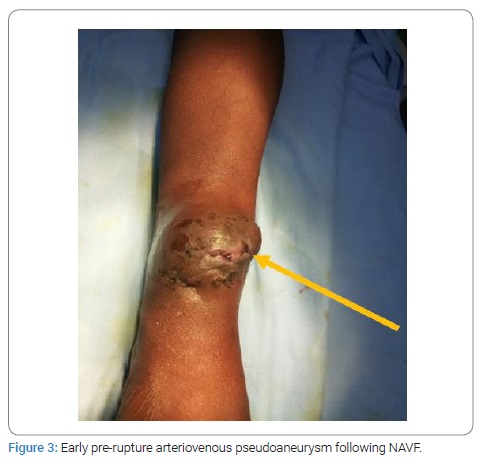
The prevalence of AVA remote from all patients carrying NAVF who developed AVA outside the upper extremity venous network used for puncture for chronic hemodialysis was 5.9% (117/2013 cases collected). The mean age was 42.30 years ± 13.31 years [extremes: 11 years and 75 years] with a sex ratio (M/F) of 2.37. The age range of 10 years to 50 years represented 68.38% (Figure 2). Sixty-one patients developing AVA had a low socioeconomic level. They were mostly unemployed or working in the informal sector (52.14%). The epidemiological aspects of our patients are summarized in (Table 1). HIV seroprevalence was 11.97%. The frequently observed cardiovascular risk factors were hypertension alone (n = 90; 84.11%) or associated with diabetes (n = 8; 7.47%). The patients’ clinical and Ultrasound Doppler characteristics were summarized in (Table 2A, 2B, and 2C). The mean pre-hospital renal clearance was 39.7 ml/min ± 1.2 ml/min [Extremes: 17 ml/min–91 ml/min]. All patients carrying NAVF who developed AVA outside the upper extremity venous network used for puncture for chronic hemodialysis were predominantly located in the left forearm (86.09%). The average rate of all patients carrying NAVF who developed AVA outside the upper extremity venous network used for puncture for chronic hemodialysis realization before the occurrence of AVA was 1.35 ± 0.087 [Extremes: 1 and 7]. Of these, 11.7% performed cardiac ultrasound coupled with Doppler, which revealed a cardiac intracavity thrombus in 6 cases. The AVAs were located at the site of the primary NAVF (n = 81; 69.23%) (Figure 3) and at the site of arterialized vein puncture (n = 36; 30.77%) as illustrated by the Valenti D classification [8] in (Table 2B). The overall mean time to AVA occurrence after NAVF was 18.14 months ± 12 months [extremes: 26 Days and 84 months].
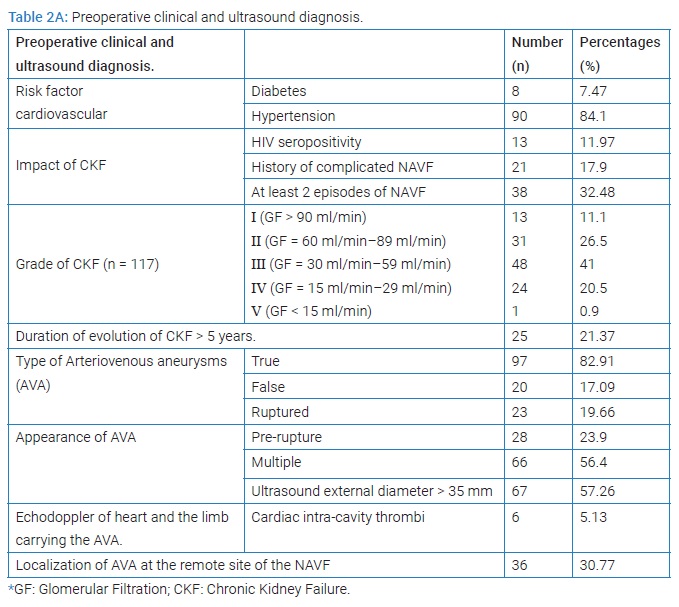
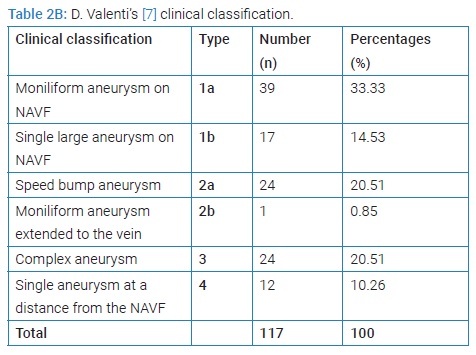
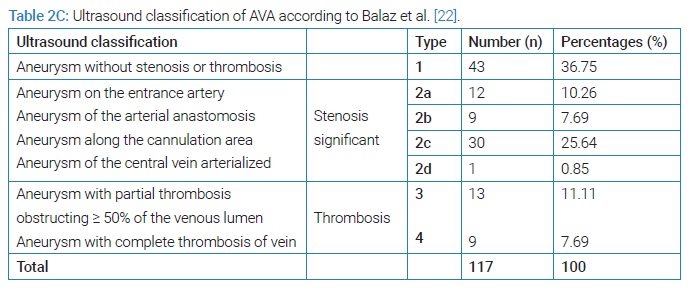
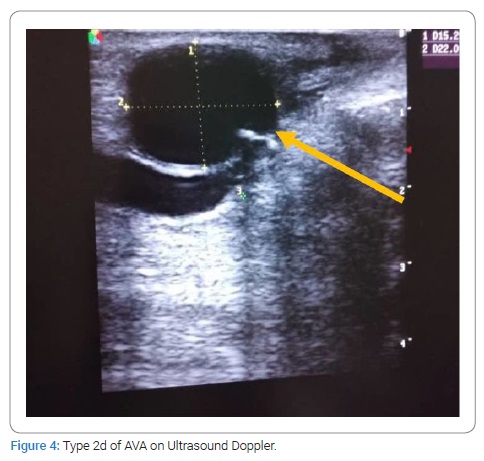
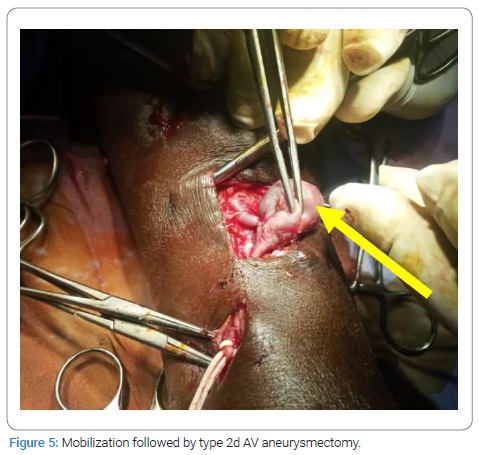
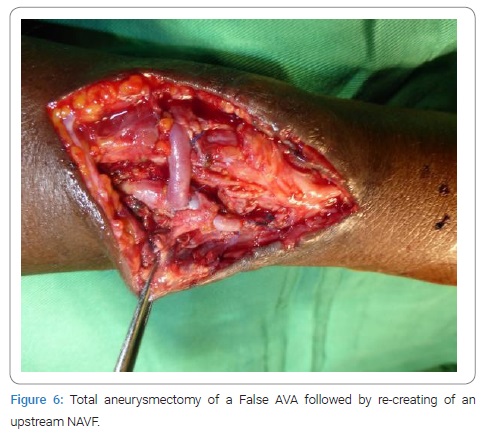
HIV- positive patients (35.71%) developed more false AVAs than HIV-negative patients (14.56%) [HR = 1.3290; CI95 (0.8922–1.9747); p = 0,049]. On native veins, false AVAs appeared early at 8.72 months ± 7.75 months [Extremes: 1 month–36 months] versus 20.08 months ± 16.8 months [Extremes: 0 month–88 months] with true AVAs (p = 0.038). The mean NAVF flow at the time of AVA occurrence was 367.87 ml/min ± 27 ml/min [0 ml and 502 ml]. False aneurysms (n = 20) and true AAV (n = 97) accounted for 17.09% and 82.91%, respectively. There was ultrasound-Doppler of the aneurysm (Figure 4), 44.44% stenosis regardless of their site, and 18.80% thrombosis of the arteriovenous aneurysm summarized in (Table 2C). The frequency of AVA thrombosis in HIV-positive patients was 27, 26% (n = 6) versus 15.53% (n = 16) of HIV-immunocompetent patients [HR = 1.4782; CI95 [0.9321–2.3442]; p = 0.014]. Fifty patients (42.74%) underwent re-operation for AVA. Clinical criteria for AVA operability were pain (100%), ultrasound External Diameter of AVA > 35 mm (n = 31; 62%), and AVA rupture (n = 19; 38%). The surgical procedures consisted of a total arteriovenous aneurysmectomy (n = 41) after mobilizing AVA (Figure 5), a first removal of the NAVF by a double proximal and distal ligation of the radial artery, and then of the arterialized vein. Then, the NAVF was immediately recontoured at a distance from the first (n = 10) (Figure 6) or secondarily (n = 31) by a cephalo-radial arterial anastomosis, termino-lateral (n = 26) and termino-terminal (n = 5). We also performed a partial aneurysmectomy with immediate re-confection of the NAVF in 9 cases. In the case of AVA surgery, the rate of NAVF conservation was 18% (n = 9), with a rate of NAVF delocalization that was 28% (n = 14). Remotely, NAVF consisted of anastomosing a reverse saphenous vein graft between the arterial ends first and then secondarily anastomosing it to the cephalic vein (n = 26). False AVAs underwent more interventions (80.61%) than 67.35% true AVAs (HR = 3.299; CI95 [1.3573–8.0183]; p = 0.00015). The mean length of hospital stay was 6.128 ± 4.4917 Hours [Extremes: 0 Hour and 48 Hours]. The mean follow-up was 4.74 patients per year [extremes: 0 year–12 years].
The loss of sight rate was 4.5%. Immediate postoperative mortality was 18% versus 10.30% for surgical abstention (HR = 1.188; CI95 [0.709–1.999]; p = 0.48). Postoperative mortality at 1 year and 5 years was 2.7% and 17.1%, respectively. We noted 17 immediate postoperative complications (34%) due to fistula hyper flow syndrome (n = 7) illustrated on fistula echo doppler, surgical wound suppuration (n = 4), AVA recurrence (n = 1), severe anemia (n = 1), and hemorrhage (n = 4).
HIV-positive patients were re-operated (n = 8; 57.14%) without distinction from HIV-negative patients (n = 14; 39.81%) [HR = 1.404; CI95 [0.7518–2.6238]; p = 0.21].
Clinical aspects and ultrasonographic aspects of arteriovenous aneurysms were found with a re-intervention rate of 41.88%, whose predictive factors were gender related to the pathological anatomical nature of arteriovenous aneurysms. Indeed, ruptured arteriovenous aneurysms (n = 20) caused more reoperations (86.96%) versus 30.85% of unruptured arteriovenous aneurysms (n = 29), HR = 5.030; CI95 [1.8297–15.3606]; p = 0.0000001. Among them, 57.14% of female patients (n = 20) had re-operated arteriovenous aneurysms versus 35.37% of males. HR = 0.6631; CI95 [0.4380–1.0038]; p = 0.029. In female patients, 50% of true arteriovenous aneurysms (n = 13) and 35% of false arteriovenous aneurysms (n = 7) resulted in re-interventions. HR = 2.25; CI95 [0.6248–8.1029]; p = 0.15. In male patients, true arteriovenous aneurysms (68.97%) were more operated on than false arteriovenous aneurysms (31.03%); HR = 3.95; CI95 [1.118–13.9565]; p = 0.00057. Male gender was the adjusted predictor of re-intervention of true arteriovenous aneurysms (HRa = 3.165; CI95 [1.2765–7.8492]; p = 0.001). In HIV-negative patients, false arteriovenous aneurysms (73.33%) resulted in more reoperations than true arteriovenous aneurysms (34.09%); HR = 39.24; CI95 [14.7696–63.7152]; p = 0.0042. Also, 62.50% of false arteriovenous aneurysms (n = 5) versus 33.30% of true arteriovenous aneurysms were re-operated in HIV-positive patients (p = 0.030). According to the pathological anatomical aspect of the arteriovenous lesion in our study, the predictor of reoperation was HIV seropositivity adjusting for false arteriovenous aneurysms HRa = 3.098; CI95 [1.3217–7.2647], p = 0.0008.
Discussion
Arteriovenous fistulas are more prone to postoperative complications such as arteriovenous aneurysms in patients debilitated by end-stage renal disease and with a low socioeconomic level [9]. In the literature [2,9,10], the prevalence of AVA as one of the main postoperative complications of NAVF is variously transmitted. The literature is heterogeneous on the risk factors for the occurrence of AVA in the case of NAVF. Among the risk factors, the quality of the NAVF [2], the surgical experience of the practitioner [11], the number of hemodialyses prior to NAVF [2], and the susceptibility of the patient to develop an aneurysm [3] are of concern. Indeed, Martin AG et al. [12], who studied the hemodynamics and maturation of the brachial AVF, noted a progressive dilatation, respectively, from 2.29 mm to 6.31 mm of the vein and from 3.76 mm to 5.39 mm of the brachial artery during the eight weeks following surgery. These vascular dilations increase with a large arteriotomy greater than 10 mm. This is because the trans-fistular flow and volume increase due to the high arterial pressure and low brachial venous pressure [13]. Despite the imminent risk of rupture of these AVAs, our patients mostly consult late, in the phase of pre-rupture syndrome or rupture of their arteriovenous aneurysm following their autologous AVF. The post- NAVF infection-type complications, thrombosis, distal limb ischemia, or venous hyper flow, although sufficiently described in the literature [2,14,15], have not been stated in the study.
AVAs are postoperative complications that occur within a variable period of time, depending on whether it is a false or true arteriovenous aneurysm following a native AVF made from an autologous vein or from a heterologous prosthesis [16]. Indeed, false AVA appears at 13.9 months on the synthetic prosthesis and at 16.6 months on the native vein, according to GS Georgiadis et al. [16]. The frequency of pseudoaneurysms among the 372 radio-cephalic arteriovenous fistulas was 6.7%, according to these authors [16]. Regardless of the location of the NAVF, whether prosthetic or auto-lateral, the incidence of false arteriovenous aneurysms varies from 2% to 10% [17,18] and from 5% to 6% for true arteriovenous aneurysms following NAVF for hemodialysis [2,19,20]. Contrarily to the data in the literature [2,18,21], false arteriovenous aneurysms are frequent in our study because of HIV infection and risk factors such as hypertension and diabetes [21,22]. The clinical diagnosis of AVA on NAVF was facilitated in all cases by D. Valenti’s classification [8]. Arteriovenous Doppler ultrasound was performed at a distance from the pain confined at the level of the aneurysm. However, this examination is the diagnostic and classification criterion of Balaz P, et al.2015 [23]. These authors [23,24] state that this vascular Doppler ultrasound is all the more essential for surgery with regard to a true aneurysm. In fact, the discovery of AVA thrombosis must lead to the per-operative complete venous thrombectomy but also to the seropositive status of the patient, as demonstrated in our study.
The importance of immediate mortality after arteriovenous anevrysmectomy cannot be emphasized. Benefits were compromised by anemic shock. Moreover, Blood conservation is especially relevant, then procuring blood and blood products and safety in developing countries with a high incidence of Hepatitis and HIV [25] lowed, procedures of blood transfusion, and increased postoperative mortality in our practice, even if patients with last stage of kidney disease still alive with chronic anemia.
In the study, the operability criteria of AVA are similar to those of a vascular aneurysm [3,24,26]. Contrary to the data in the literature [23,24,26], we operate more false aneurysms coming to the surgical emergency service at the stage of rupture, hence the low rate of conservative surgery.
Conservative treatment of AVA following NAVF is indicated when these aneurysms are true and free of stenosis. Our study explores the fistulous pathway (anastomotic artery and arterialized vein) intraoperatively by a Fogarty probe. The decision to keep the fistula or to relocate it in case of asymptomatic AVA is taken intraoperatively according to the quality of the vessels to be anastomosed. On the other hand, for symptomatic, pre-ruptured or ruptured, or infected aneurysms with ulcerated or necrotic skin, the creation of an arteriovenous fistula at the site of the aneurysm is not indicated. For ruptured fistulas, we recommend an aneurysmectomy preceded by a double arterial ligation on both sides of the fistula and the proximal end of the arterialized vein. A secondary NAVF or at a distance from the first one reduces its infection and favors skin healing. In the absence of a heterograft, we often take a saphenous vein graft that we interpose in an extra-anatomical position in the arterial circuit by a double termino-lateral and latero-lateral anastomosis, avoiding the septic focus [27].
Furthermore, we do not use the buttonhole cannulation technique, which aims to reduce the growth of the hematoma and the aneurysm [28,29]. From aneurysmorraphy via partial aneurysmectomy to total aneurysmectomy followed by re-confection of a NAVF, there is no consensus attitude towards AVA [30,31,32]. For the surgeon, it is a question of re-establishing arterial continuity in case of threat of distal ischemia, relieving the patient of his pain, correcting the aesthetic defect, and preserving the chances of regular hemodialysis. Thus, recourse to heterografts [31,32] and autografts such as saphenous veins [27] is indispensable in the case of a giant or voluminous aneurysm. Indeed, the termino-lateral veno-arterial anastomosis upstream of the first NAVF ensures a good trans-fistular volume and a flow rate necessary to ensure early hemodialysis. However, preventing bleeding, infection, and distal limb function (ischemia) seems to guide every vascular surgeon, as all surgical techniques have both advantages and disadvantages.
The independent variables (age, sex, HIV status, and pathologic anatomic appearance of the arteriovenous aneurysm) used in our study were accepted by the researchers and are considered risk factors for re-intervention of arteriovenous aneurysms following native arteriovenous fistulas [33]. However, their performance in predicting re-intervention is not always ideal, so we suggest that in another large retrospective cohort, adjusted association strengths should be investigated, and new, more valuable predictors should be identified. Despite this, the predictor of re-intervention, according to our study’s pathological anatomical aspect of the arteriovenous lesion, was HIV seropositivity adjusting for false arteriovenous aneurysms.
In doing so, the limitations of our study appear to be the small size of the subgroups formed and not the overall sample size. Possible confounding biases (such as preoperative ruptures of arteriovenous aneurysms) do not alter the effect of known risk factors for aneurysms such as HIV status alone [34] or associated with pseudoaneurysm [14,18,35], postoperative hemorrhage [36], or the skill of the vascular surgeon making the native arteriovenous fistula in insufficient renal patients. Furthermore, there are some limitations of our study regarding selection bias. Our patients excluded for missing data could be adjusted within the study.
Conclusion
Aneurysms on native arteriovenous fistula are frequent. Their clinical and ultrasonographic diagnosis is easy and well-codified. In our clinical experience, delays in receiving treatment are so frequent that they increase mortality and immediate postoperative complications. A high rate of re-operation for conservation or removal of the native arteriovenous fistula still compromises the survival of these severely renal insufficient patients. In order to improve the vital prognosis of these insufficient renal patients subjected to several surgical interventions and to identify the patients at risk of developing postoperative complications, it is imperative to determine the predictive factors of reintervention, which are the sex and the HIV status related to the various anatomical, pathological types of arteriovenous aneurysms.
Declaration and Ethical Approval
We have no conflict of interest with the patients, the biological laboratories involved in this work, or the Scientific Direction of the Research of our practice hospital.
Ethical approval and consent to participate: We contribute to current scientific, clinical, or social knowledge through this article. By this article, we mean to protect the rights and promote the well-being of participants.
Consent to publication: All co-authors of this article have given permission for publication.
Availability of data and material: We are available to justify our database summarized in an “Excel” database obtained from the patient follow-up logbook.
Conflict of Interest
The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Padberg FT Jr, Calligaro KD, Sidawy AN. Complications of artériovenous hemodialysis access: recognition and management. J Vasc Surg. 2008;48(5 Suppl):55S–80S.
- Al-Jaishi AA, Liu AR, Lok CE, Zhang JC, Moist LM. Complications of the arteriovenous fistula: a systematic review. J Am Soc Neph. 2017;28(6):1839–1850.
- Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48 Suppl 1:S176–247.
- Saeed F, Kousar N, Sinnakirouchenan R, Ramalingam VS, Johnson PB, Holley JL. Perte de sang par fistule artério-veineuse: Rapport de cas et revue de la littérature. Int J Nephrol. 2011;2011:1–6.
- Jiber H, Naouli H, Bouarhroum A. Prise en charge des anévrismes sur fistules artério- veineuses pour hémodialyse chronique. J MalVasc. 2015;40(5):318.
- Brescia MJ, Cimino JE, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275(20):1089–1092.
- Zierler RE, Jordan WD, Lal BK, Mussa F, Leers S, Fulton J, et al. The Society for Vascular Surgery practice guidelines on follow-up after vascular surgery arterial procedures. J Vasc Surg. 2018;68(1):256–284.
- Valenti D, Mistry H, Stephenson M. A novel classification system for autogenous arteriovenous fistula aneurysms in renal access patients. Vasc Endovascular Surg. 2014;48(7-8):491–496.
- Kalfat T, Ghedira F, Elleuch N, Kaouel K, Mrad MB, Miri R, et al. Prise en charge des complications des accès d’hémodialyse. Cardiol Tunis. 2013; 9(1):20–25.
- Ahn SY, So YH, Choi YH, Jung IM, Chung JK. Endovascular recanalization of a thrombosed native arteriovenous fistula complicated with an aneurysm: technical aspects and outcomes. Korean J Radiol. 2015;16(2):349–356.
- Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40(3):611–622.
- Martin AG, Grasty M, Lear PA. Haemodynamics of brachial arteriovenous fistula development. J Vasc Access. 2000;1(2):54–59.
- Georgakarakos EI, Kapoulas KC, Georgiadis GS, Tsangaris AS, Nikolopoulos ES, Lazarides MK. An overview of the hemodynamic aspects of the blood flow in the venous outflow tract of the arteriovenous fistula. J Vasc Access. 2012;13(3):271–278.
- Bachleda P, Utíkal P, Zadrazil J, Grosmanová T. [Aneurysm as a complication of arteriovenous anastomoses for hemodialysis]. Rozhl Chir. 1998;77(12):541–544.
- Belli S, Yabanoglu H, Aydogan C, Parlakgumus A, Yildirim S, Haberal M. Surgical interventions for late complications of arteriovenous fistulas. Inter Surg. 2014;99(4):467–474.
- Georgiadis GS, Lazarides MK, Panagoutsos SA, Kantartzi KM, Lambidis CD, Staramos DN, et al. Surgical revision of complicated false and true vascular access–related aneurysms. J Vasc Surg. 2008;47(6):1284–1291.
- Yasim A, Kabalci M, Eroglu E, Zencirci B. Complication of hemodialysis graft: anastomotic pseudoaneurysm: a case report. Transplant Proc. 2006;38(9):2816–2818.
- Georgiadis GS, Lazarides MK, Lambidis CD, Panagoutsos SA, Kostakis AG, Bastounis EA, et al. Use of short PTFE segments ( <6 cm) compares favorably with pure autologous repair in failing or thrombosed native arteriovenous fistulas. J Vasc Surg. 2005;41(1):76–81.
- Palder SB, Kirkman RL, Whittemore AD, Hakim RM, Lazarus JM, Tilney NL. Vascular access for hemodialysis. Patency rates and results of revision. Ann Surg. 1985;202(2):235–239.
- Ballard JL, Bunt TJ, Malone JM. Major complications of angioaccess surgery: Am J Surg. 1992;164(3):229–232.
- Cavallaro G, Taranto F, Cavallaro E, Quatra F. Vascular complications of native arteriovenous fistulas for hemodialysis: role of microsurgery. Microsurgery. 2000;20(5):252–254.
- Jankovic A, Donfrid B, Adam J, Ilic M, Djuric Z, Damjanovic T, et al. Arteriovenous fistula aneurysm in patients on regular hemodialysis: prevalence and risk factors. Nephron Clin Pract. 2013;124(1–2):94–98.
- Balaz P, Björck M. True aneurysm in autologous hemodialysis fistulae: definitions, classification and indications for treatment. J Vasc Access. 2015;16(6):446–453.
- Baláž P, Rokošnỳ S, Bafrnec J, Whitley A, O’Neill S. Repair of aneurysmal arteriovenous fistulae: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2020;59(4):614–623.
- Seely AJE, Ivanovic J, Threader J, Al-Hussaini A, Al-Shehab D, Ramsay T, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90(3):936–942.
- Al-Thani H, El-Menyar A, Al-Thani N, Asim M, Hussein A, Sadek A, et al. Characteristics, management, and outcomes of surgically treated arteriovenous fistula aneurysm in patients on regular hemodialysis. Ann Vasc Surg. 2017;41:46–55.
- Malekpour F, Sutton J, Kirkwood M. Late development of a large arteriovenous fistula with aneurysm in an in situ vein bypass graft. Ann Vasc Surg. 2019;57:275.e1–e275.
- Marticorena RM, Hunter J, Macleod S, Petershofer E, Dacouris N, Donnelly S, et al. The salvage of aneurysmal fistulae utilizing a modified buttonhole cannulation technique and multiple cannulators. Hemodial Int. 2006;10(2):193–200.
- Jennifer M, Mac R. Doit-on utiliser la canulation en boutonnière des fistules artério- veineuses? Rein 360. 2020;1(5):322–325.
- Piccolo III C, Madden N, Famularo M, Domer G, Mannella W. Partial aneurysmectomy of venous aneurysms in arteriovenous dialysis fistulas. Vasc Endovasc Surg. 2015;49(5–6):124–128.
- Berard X, Brizzi V, Mayeux S, Sassoust G, Biscay D, Ducasse E, et al. Salvage treatment for venous aneurysm complicating vascular access arteriovenous fistula: use of an exoprosthesis to reinforce the vein after aneurysmorrhaphy. Eur J Vasc Endovasc Surg. 2010;40(1):100–106.
- Hossny A. Partial aneurysmectomy for salvage of autogenous arteriovenous fistula with complicated venous aneurysms. J Vasc Surg. 2014;59:1073–1077.
- Can A, Gross BA, Du R. The natural history of cerebral arteriovenous malformations. Handb Clin Neurol. 2017;143:15–24.
- Bayley AC. Surgical pathology of HIV infection: lessons from Africa. Br J Surg. 1990;77(8):863–868.
- Marks C, Kuskov S. Pattern of arterial aneurysms in acquired immunodeficiency disease. World J Surg. 1995;19(1):127–132.
- Stapf C, Mohr JP, Pile-Spellman J, Sciacca RR, Hartmann A, Schumacher HC, Mast H. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. 2002;73(3):294–298.
Keywords
Arteriovenous aneurysms; Surgery; Côte d’Ivoire, Fistulas; Outcomes
Cite this article
Ayegnon KG, Diby KF, Abro S, Choho MCC, Gnaba LA, Amani KHA, et al. Epidemio-clinical profile and treatment of aneurysms on native arteriovenous fistula waiting for chronic hemodialysis in Ivory Coast. Clin Surg J. 2023;4(1):1–9.
Copyright
© 2023 Ayegnon Kouakou Grégoire. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).










