Severe Atypical Resistant Form of Ocular Toxoplasmosis Using Combined Therapy: Case Report
* Boris Bajaire-Gomez;
Haishel Sierra-Mejía;
Sara Martinez-Roman;
-
* Boris Bajaire-Gomez: IPS Bajaire, Bogotá, Colombia; Instituto Oftalmológico Bajaire - Global Medical Center, Bogotá, Colombia.
-
Haishel Sierra-Mejía: IPS Bajaire, Bogotá, Colombia.
-
Sara Martinez-Roman: IPS Bajaire, Bogotá, Colombia.
-
Dec 15, 2022 |
-
Volume: 3 |
-
Issue: 4 |
-
Views: 3687 |
-
Downloads: 2036 |
Abstract
Ocular toxoplasmosis, an infection caused by Toxoplasma gondii, is the most common cause of infectious posterior uveitis in immunocompetent patients. This paper aims to report the case of a woman with retinitis associated with ocular toxoplasmosis and an intravitreal dexamethasone implant.
A 30-year-old woman was observed during a medical appointment; she consulted for myodesopsias in her left eye. We found uveitis, vasculitis, vitreous opacities, and epiretinal membrane during the initial examination. Blood tests for HIV, Hepatitis, and Epstein Barr were negative, while Toxoplasma antibodies (IgG and IgM) were positive. We performed a vitrectomy in the affected eye, followed by combined treatment with Trimethoprim-Sulfamethoxazole, clindamycin, and prednisolone. Shortly after, macular edema was observed, and we performed an intravitreal dexamethasone implant. Two months later, she developed active retinitis and retinal detachment, for which it was necessary to perform a vitrectomy with silicone oil tamponade. One month later, the infection was controlled, visual acuity improved and stabilized, and the retina was attached.
There is a controversy about the route, initiation, and timing of adjunctive corticoid therapy. In addition, evidence for intravitreal dexamethasone implant is still scarce in the course of infectious uveitis. More studies are needed to define longer-term visual and anatomic results.
Abbreviations
T. gondii: Toxoplasma gondii, BCVA: Best-Corrected Visual Acuity; HIV: Human Immunodeficiency Virus; OCT: Optical Coherence Tomography; ERM: Epiretinal Membrane; WBC: White Blood Cells; PCR: Polymerase Chain Reaction; Ab: Antibody; PPV: Pars Plana Vitrectomy; HCMV: Cytomegalovirus; IgG: Immunoglobulin G; IgM: Immunoglobulin M; Anti-LA: Single-stranded DNA Binding Proteins; Anti-RNP: Anti- Ribonucleoprotein; Anti-RO: Anti-Sjögren’s Syndrome Related Antigen A; Anti-SM: Anti-Smith Antibodies; ANA: Anti-Nuclear Antibodies; ACPAs: Anti-Citrullinated Protein Antibodies
Introduction
Toxoplasma gondii (T. gondii), an obligate protozoan parasite that can lead to acute or chronic infections, can cause ocular toxoplasmosis in humans [1]. Humans acquire the infection by ingesting the parasite from raw or undercooked meat, direct contact or inhalation of oocysts, organ transplantation from infected donors, and transplacental transmission [1].
Ocular toxoplasmosis is the most common cause of infectious posterior uveitis. The prevalence and clinical course of the disease vary significantly regarding different regions and socioeconomic factors [2–8]. Retina and choroid may become infected by the parasite several weeks to several years after the initial systemic illness [1].
The prevalence of ocular toxoplasmosis and the sources of infection vary depending on the geographic region, climate, eating habits, and hygienic habits of a population. In Colombia, 47% of the population has antibody titers for Toxoplasma gondii; it is estimated that 1,000,000 people have retinochoroidal scars; the estimated incidence of ocular toxoplasmosis in Colombia, in immunocompetent patients is three new cases per 100,000 inhabitants per year [28]. The presentation of ocular toxoplasmosis tends to be more severe in South American territories compared to Europe. The difference can be attributed to the South American strains being more virulent type I/III, unlike the European type II. In European countries, for example, Turkey, the seroprevalence of toxoplasmosis is 30%, and ocular toxoplasmosis represents 7.2% of all cases of posterior uveitis in Istanbul [6].
Lesions can be solitary, multiple, or satellite to a pigmented retinal scar [9,10]; the optic nerve head can also be involved [11]. However, clinical diagnosis may be even more challenging in immunocompromised patients, as bilateral involvement, large areas of retinal necrosis, and multifocal chorioretinitis can be found [12–16]. Atypical ocular toxoplasmosis presentations include punctate outer retinal toxoplasmosis, retinal vasculitis, retinal vascular occlusions, and rhegmatogenous with serous retinal detachments, among others [15].
Therapy must target both the parasite and host immunity. Typical regimens include Pyrimethamine/Sulfadiazine or Trimethoprim-Sulfamethoxazole and Prednisolone as triple therapy, with the addition of Clindamycin in quadruple treatment for at least 4 weeks–6 weeks [17]. Some authors have reported oral Prednisolone dosages ranging from 1.0 mg/kg/day to 1.5 mg/kg/day, up to a daily maximum of 20 mg/day [17]. However, corticosteroid therapy in patients with ocular toxoplasmosis varies from not using them at all to starting them within three days or a week after anti-parasitic treatment [18]. In addition, other treatments may be necessary, such as intravitreal injections of Clindamycin with or without dexamethasone and posterior vitrectomy with silicone oil tamponade [19].
The current study aims to report the course of an atypical case of ocular toxoplasmosis that did not respond to traditional treatment and worsened after the suspension of oral corticoid therapy and intravitreal Dexamethasone implant.
Case Presentation
A 30-year-old woman who resides in Bogota, Colombia, traveled to Turkey 10 months ago and consulted our clinic for one month about the evolution of myodesopsias in her left eye. Our initial examination showed that her Best-Corrected Visual Acuity (BCVA) was 20/20 in her right eye and 20/80 in her left eye. In the fundus of the left eye, there was evidence of vitreous opacification of the vitreous cavity, healthy papilla, and ghost vessels in the upper sector with inflammatory presence. Posterior uveitis was suspected, so we indicated one drop four times a day of Prednisolone at 1%.
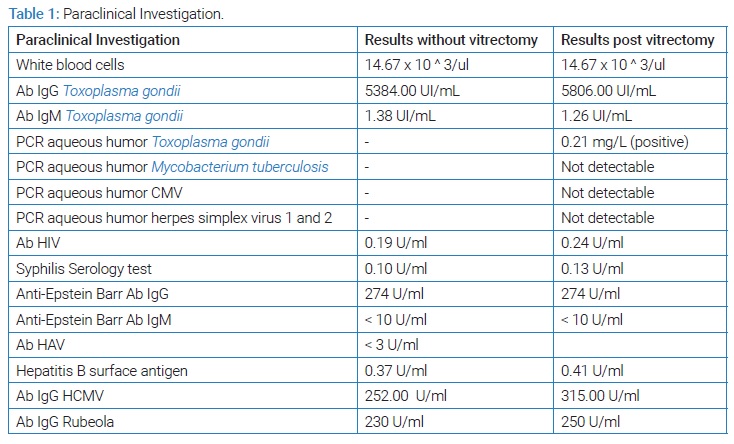
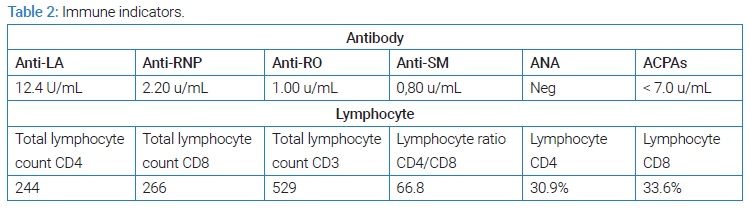
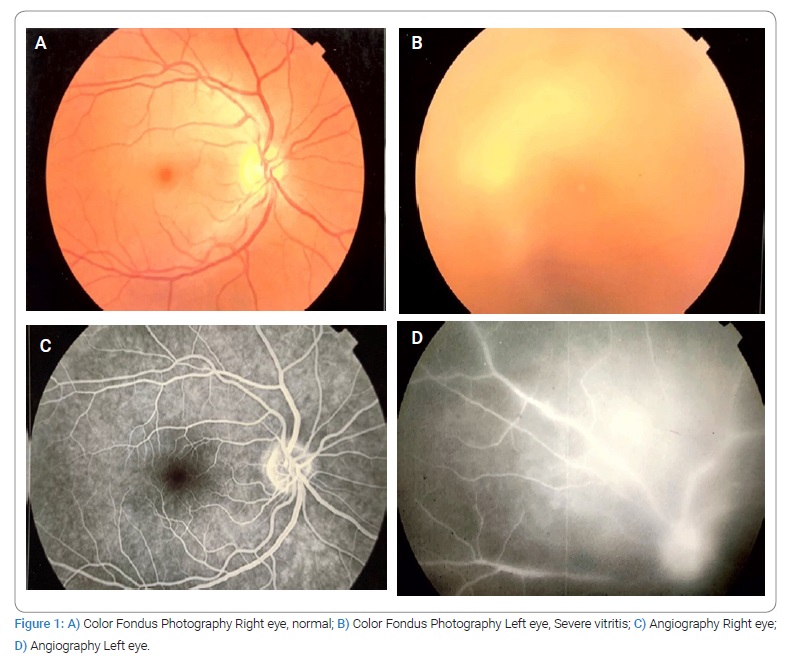
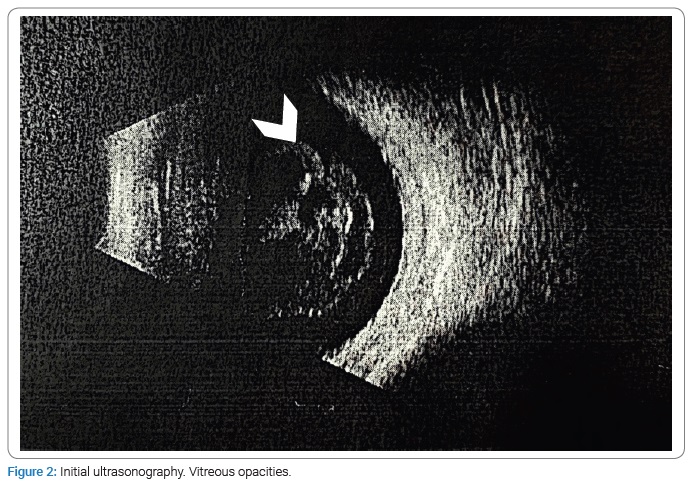
Given the high probability of posterior uveitis, immunodeficiency and infection by opportunistic microorganisms were ruled out. Blood tests showed Toxoplasma IgG antibodies (+) (5384 UI/mL) and IgM antibodies (+) (1.38 UI/mL) (Table 1,Table 2). In the angiography, it is impossible to demonstrate details of the fundus due to media opacity; however, blood vessels with staining of their walls and late leaks of the dye are observed, with findings highly suggestive of uveitis and retinal vasculitis (Figure 1). Moreover, severe vitreous opacities of low reflectivity, without evidence of vitreous detachment, were reported in the ultrasound (Figure 2).
Results
A Pars Plana Vitrectomy (PPV) in the left eye was performed, and a sample of the vitreous humor was taken to carry out diagnostic tests. Within the intraoperative findings, multiple vitreous opacities and retinal vascular sheathing in temporal and superior vascular arcades. After surgery, we found vitritis with no retinal detachment, so we started a combined oral treatment with Trimethoprim 160 mg -Sulfamethoxazole 800 mg twice daily, Clindamycin 300 mg four times a day, and Prednisolone 5 mg once a day. Optical Coherence Tomography (OCT) showed a hyper-reflective macular lesion and a foveal Epiretinal Membrane (ERM) with a macular thickness of 317 µm (Figure 3A).
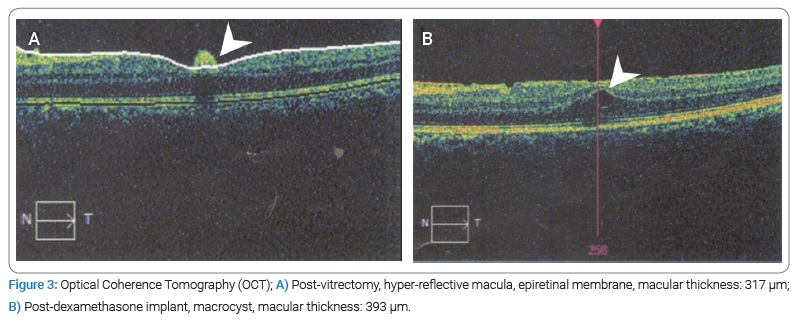
After four weeks of antibiotic treatment with Trimethoprim 160 mg -Sulfamethoxazole 800 mg twice daily, and Clindamycin 300 mg four times a day without clinical improvement, we decided to perform an intravitreal Dexamethasone 700 µg implant in the left eye. However, the visual acuity continued to deteriorate during the following four weeks, going from 20/40-2 to 20/70-2. Macular thickness increased up to 393 μm, and macro- and microcysts appeared in OCT (Figure 3B).
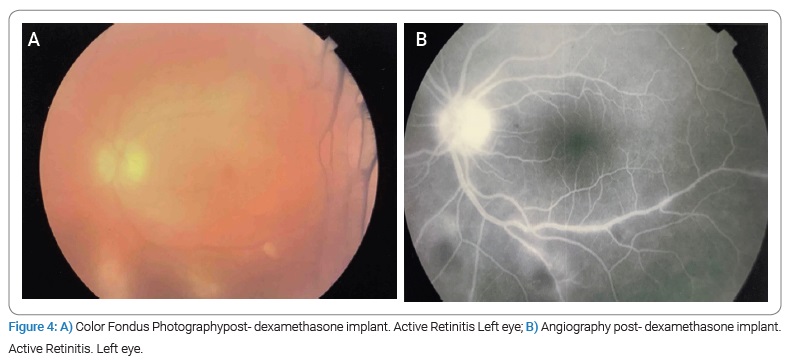
Two months after the implant, macular thickness increased up to 434 µm, suggesting active retinitis (Figure 4).
We initiated a combined scheme with 40 mg intramuscular Methylprednisolone, one drop three times a day of diclofenac, and Trimethoprim 160 mg -Sulfamethoxazole 800 mg twice daily, Clindamycin 300 mg four times a day for another six weeks. At this time, blood tests showed Neutrophilic leukocytosis (WBC: 14,670, neutrophilia: 80%) and higher levels of Toxoplasma-IgG (5806 UI/mL), with negative C-reactive protein (0.21 mg/L) (Table 1). Polymerase Chain Reaction (PCR) analysis of aqueous humor confirmed the presence of T. gondii; mycobacteria, cytomegalovirus, and herpes simplex virus 1 and 2 PCR were negative, the Elecsysnm® Toxo IgG IgG avidity test was also performed, which yields a high percentage (90%).
After a month of the last antibiotic scheme, BCVA decreased up to 20/100. The vitreous had mild cellularity (flare +3) according to standardized rating scales for uveitis, so we decided to give an intravitreal injection of Clindamycin 1 mg, followed by a subconjunctival dose of Dexamethasone 8 mg after vision decreased to hand motions a week later. Unfortunately, despite many therapeutic efforts, vitritis was still evident on ultrasound.
Given the inadequate response to treatment and the persistence of symptoms, the patient consulted another uveitis specialist, who stopped corticoid treatment gradually. Shortly after, vision decreased rapidly in one month, and the patient came back to our clinic, where we restarted therapy with oral Prednisolone 1 mg/kg, topic prednisolone one drop four times a day, Pyrimethamine 75 mg a day for three days, followed by 35 mg every week and Folic acid 25 mg a day.
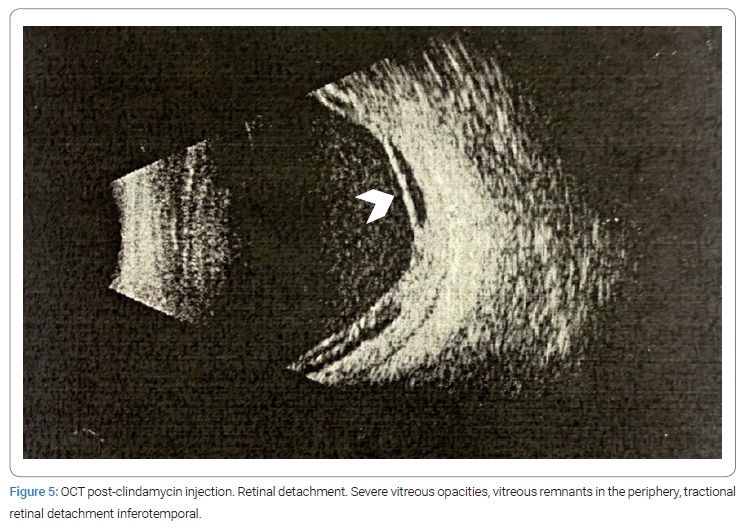
One month after taking the Clindamycin injection, no improvement in visual acuity was evident. The ultrasound showed retinal detachment (Figure 5), so we performed a second vitrectomy. Within the intraoperative findings, inferior fibrosis was evident, and total retinal detachment. An inferior relaxing retinectomy was performed, perfluorocarbon was injected, endolaser was performed on the posterior edge of the retinectomy and in lower quadrants, and finally, injection of silicone oil was fully applied to the retina. After this, visual acuity slowly improved up to 20/60, the retina was attached entirely, and infection was controlled (Figure 6).
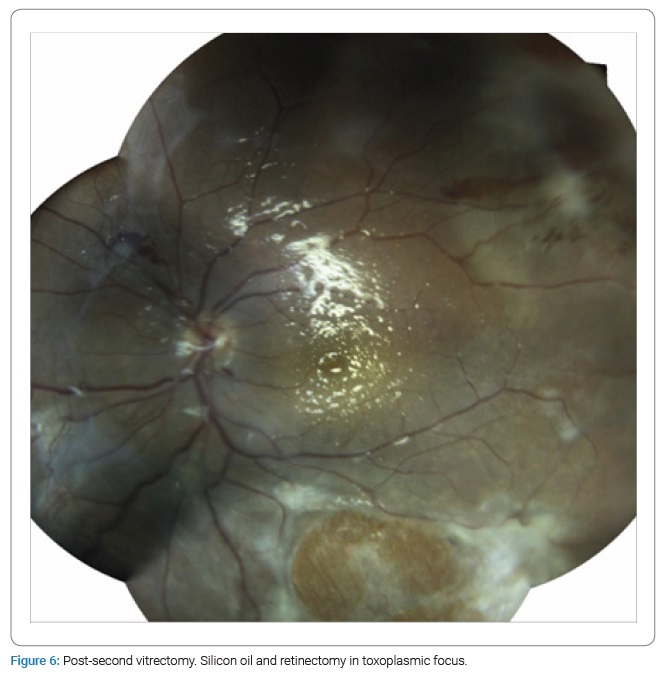
At present, the patient is in annual check-ups; on the last May of 2022, BCVA was evident in the left eye of 20/60, which is a visual acuity better than expected, given the complications and sequelae of severe inflammation. In addition, there has been no recurrence in a fundus with a fully engaged retina with 360-degree peripheral gliosis bands and multiple mainly inferior laser scars with gliosis surrounding retinectomy, without signs of active retinitis.
Discussion
The development of a fulminant and resistant form of ocular toxoplasmosis has been reported. In a case like this, treatment should focus on three aspects: to block the multiplication of the parasite during active chorioretinitis, to stop the necrotizing inflammation, and to minimize damage over the retina and optic nerve [20].
The most used therapeutic regimen for ocular toxoplasmosis consists of Pyrimethamine and Sulfadiazine, plus corticosteroids. In selected cases, Trimethoprim/Sulfamethoxazole plus oral Prednisolone or intravitreal Clindamycin and Dexamethasone injections may be alternative treatment options [21].
When used, corticoids are usually started simultaneously or 24 hours–72 hours after initiating antibiotic therapy [20,22–24]. However, whether given systemically or periocularly, corticosteroids should always be administered with a shield of antiparasitic drugs [25].
In a cross-sectional survey of uveitis specialists, 17% responded that they initiated corticosteroid management in immunocompetent patients regardless of clinical findings [33]. The other physicians refer to the use of corticosteroid therapy in specific cases, like vitreous inflammatory severe reaction (71%). The American Academy of Ophthalmology concluded that there was no evidence from randomized controlled trials to support or refute the effectiveness of adjunctive steroid therapy for treating ocular toxoplasmosis [18]. However, the use of this therapy is supported by the knowledge that the use of corticosteroids suppresses inflammation and therefore decreases damage to ocular tissue [17].
Nevertheless, there is a long-lasting controversy around the use of corticosteroids in ocular toxoplasmosis. Some authors [22] have reported that iatrogenic immunosuppression induced by corticosteroid monotherapy may lead to an aggressive course and severe complications of ocular toxoplasmosis, such as retinochoroiditis. Conversely, as visual damage in toxoplasmosis has been attributed to direct inflammation by the parasite, corticosteroids could be beneficial given their anti-inflammatory properties [26]. Furthermore, intravitreal dexamethasone implants may be safe and effective alternatives in macular edema secondary to uveitis from several infectious etiologies, toxoplasmosis included [27].
In this case, we appreciate the two sides of the same coin: when oral corticoid doses were decreased and treatment was stopped, visual acuity diminished dramatically, but when we restarted prednisone and injected intravitreal dexamethasone, vitritis worsened and evolved to necrotizing retinitis with secondary retinal detachment.
Seemingly, silicone oil tamponade was the best treatment for secondary complications of the infection as well as the way to control the infection. Indeed, although retinal detachment is a relatively rare complication of ocular toxoplasmosis, previous reports suggest that once this condition appears, it may require several therapies, including multiple surgical procedures and systemic and intravitreal medications [19].
Moreover, it is very important to identify if uveitis is of infectious etiology or not since it allows timely treatment and offers a better prognosis for the patient. Uveitis syndromes can be diagnosed by a combination of medical history, physical examination, laboratory, and imaging [29,30]. However, there are clinical dilemmas in which it is difficult to determine the etiology, such as atypical or severe presentations of the disease, among others. A diagnostic and therapeutic vitrectomy is useful in these cases [29].
Based on a retrospective study in which seventy-five patients (84 eyes) with posterior uveitis who underwent PPV were selected and followed up retrospectively from 2005 to 2009, it was evidenced that visual acuity and inflammatory activity showed marked improvement after PPV, with an improvement in visual acuity from 20/200 to 20/80 after one year (Snellen, P ≤ 0.001) [30].
Vitrectomy allows clarification of the posterior cavity, and some specialists believe it can favorably influence the evolution of uveitis, although this point is debatable [30]. In a case series of 8 patients who underwent vitrectomy for toxoplasmosis, a patient with active retinochoroiditis was identified and treated with antibiotics and corticosteroids for many months. However, the vitreous opacities and inflammation persisted, and due to the marked inflammation and decreased visual acuity, a vitrectomy was performed. No recurrence was identified in a 20-year follow-up, according to the previous case, and in the case of our patient, it can be inferred that vitrectomy is an excellent therapeutic and diagnostic method in severe cases of vitritis, given the marked improvement in visual acuity reported, the decrease in recurrence and the inflammatory process, as well as the question that it is probably involved in removing factors that could be involved in inflammatory processes and recurrence [31].
Regarding the route of infection, it can be deduced that it was a recently acquired ocular toxoplasmosis, that is, less than a year of contagion; because both IgG and IgM antibodies were positive, IgM antibodies became negative after a year of infection [32]. However, having an avidity test more outstanding than 30%, it can be concluded that the infection was in a period higher than four months. Therefore, we can infer that there is the same risk of acquiring the infection in Colombia and on the trip reported by the patient to Turkey, in which there is no notion of exposure to any identifiable risk factor. However, despite that, it is within the estimated window period for infection (between 4 months and a year).
Given the high prevalence of this disease in Colombia and Turkey, it is difficult to determine the site of infection. However, due to the severity of the disease presentation, it can be concluded that it was a highly virulent infection (type I/III and atypical), which is more common in South American territories.
Conclusions
The goal of treating ocular toxoplasmosis is to reduce the risk of visual loss and the multiplication of the parasite during the active state, the inflammation, and the sequelae produced by it. It is usually managed with antibiotic therapy; however, in the case of severe vitritis and poor response to antibiotics, it is necessary to evaluate and use other less conventional management.
In the presentation and management of our patient’s disease, we identified various controversies, firstly, the performance of posterior vitrectomy as a therapeutic and diagnostic method. As we highlighted in the literature and as evidenced in the reported case, posterior vitrectomy in cases of severe vitritis is extremely useful to rule out complications and severe lesions that are not possible to visualize due to the opacity of the media, as well as to make an opportune diagnosis and treatment directed to the etiological agent.
Secondly, regarding corticosteroid therapy, as mentioned above, there is controversy about the route, initiation, and timing of adjunctive corticoid therapy. In addition, evidence for intravitreal dexamethasone implant is still scarce in infectious uveitis. More evidence and more studies are needed to determine the resolution time, the improvement of visual acuity, and the response to therapy since it is not known, as well as studies that establish a therapeutic regime to avoid risks and iatrogenicity with its use.
Acknowledgments
Statement of ethics: Ethical approval is not required for this study in accordance with local or national guidelines. The patient signed a written informed consent form in which he agreed to the use of his clinical data and photographs for the publication of this case report. Data anonymity was always ensured.
Data availability statement: All data generated or analyzed during this case report are included in this article. Further inquiries can be directed to the corresponding author.
Declaration of interests: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding sources: None of the authors have any financial or proprietary interest in any material or method mentioned in this article.
Author contributions: Boris Bajaire developed the original idea, revised the manuscript, supervised the treatment, and is the guarantor. Haishel Sierra helped with follow-ups with the patient and the data acquisition and prepared the manuscript. Sara Martinez, data acquisition, writing, proofreading, and manuscript production.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- McLeod R, Cohen W, Dovgin S, Finkelstein L, Boyer KM. Human Toxoplasma infection. In: Weiss LM, Kim K, editors. Human Toxoplasma infection. 3rd ed. London: Elsevier; 2020;117–227.
- Vasconcelos-Santos DV. Ocular manifestations of systemic disease: toxoplasmosis. Curr Opin Ophthalmol. 2012;23(6):543–550.
- Rothova A. Ocular manifestations of toxoplasmosis. Curr Opin Ophthalmol. 2003;14(6):384–388.
- Maenz M, Schlüter D, Liesenfeld O, Schares G, Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res. 2014;39:77–106.
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136(6):973–988.
- Tugal-Tutkun I, Corum I, Otük B, Urgancioglu M. Active ocular toxoplasmosis in Turkish patients: a report on 109 cases. Int Ophthalmol. 2005;26(6):221–228.
- Talabani H, Mergey T, Yera H, Delair E, Brézin AP, Langsley G, et al. Factors of occurrence of ocular toxoplasmosis. A review. Parasite. 2010;17(3):177–182.
- Kijlstra A, Petersen E. Epidemiology, pathophysiology, and the future of ocular toxoplasmosis. Ocul Immunol Inflamm. 2014;22(2):138–147.
- Nussenblatt RB, Belfort Jr R. Ocular toxoplasmosis. An old disease revisited. JAMA. 1994;271(4):304–307.
- Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004;137(1):1–17.
- Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye. 2007;21(6):746–751.
- Moshfeghi DM, Dodds EM, Couto CA, Santos CI, Nicholson DH, Lowder CY, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004;111(4):716–725.
- Elkins BS, Holland GN, Opremcak EM, Dunn Jr JP, Jabs DA, Johnston WH, et al. Ocular toxoplasmosis misdiagnosed as cytomegalovirus retinopathy in immunocompromised patients. Ophthalmology. 1994;101(3):499–507.
- Fardeau C, Romand S, Rao NA, Cassoux N, Bettembourg O, Thulliez P, et al. Diagnosis of toxoplasmic retinochoroiditis with atypical clinical features. Am J Ophthalmol. 2002;134(2):196–203.
- Smith JR, Cunningham ET. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol. 2002;13(6):387–392.
- Singer MA, Hagler WS, Grossniklaus HE. Toxoplasma gondii retinochoroiditis after liver transplantation. Retina 1993;13(1):40–45.
- Rush R, Sheth S. Fulminant toxoplasmic retinochoroiditis following intravitreal triamcinolone administration. Indian J Ophthalmol. 2012;60(2):141–143.
- Jasper S, Vedula SS, John SS, Horo S, Sepah YJ, Nguyen QD. Corticosteroids as adjuvant therapy for ocular toxoplasmosis. Cochrane Database Syst Rev. 2017;1(1):CD007417.
- Faridi A, Yeh S, Suhler EB, Smith JR, Flaxel CJ. Retinal detachment associated with ocular toxoplasmosis. Retina. 2015;35(2):358–363.
- Casoy J, Nascimento H, Silva LMP, Fernández-Zamora Y, Muccioli C, Dias JR de O, et al. Effectiveness of treatments for ocular toxoplasmosis. Ocul Immunol Inflamm. 2020;28(2):249–255.
- Park YH, Nam HW. Clinical features and treatment of ocular toxoplasmosis. Korean J Parasitol. 2013;51(4):393–399.
- Oray M, Ozdal PC, Cebeci Z, Kir N, Tugal-Tutkun I. Fulminant ocular toxoplasmosis: the hazards of corticosteroid monotherapy. Ocul Immunol Inflamm. 2016;24(6):637–646.
- Commodaro AG, Belfort RN, Rizzo LV, Muccioli C, Silveira C, Burnier MN, et al. Ocular toxoplasmosis: an update and review of the literature. Mem Inst Oswaldo Cruz. 2009;104(2):345–350.
- Hosseini SM, Abrishami M, Mehdi ZM. Intravitreal clindamycin in the treatment of unresponsive zone one toxoplasmic chorioretinitis: a case report. Iran Red Crescent Med J. 2014;16(11):e15428.
- Bosch-Driessen EH, Rothova A. Sense and nonsense of corticosteroid administration in the treatment of ocular toxoplasmosis. Br J Ophthalmol. 1998;82(8):858–860.
- Cerqueira Lima GS, Saraiva PGC, Saraiva FP. Current therapy of acquired ocular toxoplasmosis: a review. J Ocul Pharmacol Ther. 2015;31(9):511–517.
- Fonollosa A, Llorenç V, Artaraz J, Jimenez B, Ruiz-Arruza I, Agirrebengoa K, et al. Safety and efficacy of intravitreal dexamethasone implants in the management of macular edema secondary to infectious uveitis. Retina. 2016;36(9):1778–1785.
- Faciolince LA, López de Mesa C, de-la-Torre A. Toxoplasmosis ocular en Colombia: 10 años de aportes investigativos. Rev SCO. 2018; 51(1):16–28.
- Jeroudi A, Yeh S. Diagnostic vitrectomy for infection uveitis. Int Ophthalmol Clin. 2014;54(2):173–197.
- Oahalou A, Schellekens PAWJF, de Groot-Mijnes JD, Rothova A. Diagnostic pars plana vitrectomy and aqueous analyses in patients with uveitis of unknown cause. Retina (Philadelphia, Pa.). 2014;34(1):108–114.
- Bovey EH. Usefulness of vitrectomy in the treatment of ocular toxoplasmosis. Int J Med Sci. 2009;6(3):139.
- Silveira C. A maior Epidemia do mundo. Toxoplasmose dúvidas e controv 1st ed erechim edifapes. 2002;79–82.
- Kim SJ, Scott IU, Brown GC, Brown MM, Ho AC, Ip MS, et al. Interventions for toxoplasma retinochoroiditis: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120(2):371–378.
Keywords
Toxoplasma gondii; Oculartoxoplasmosis; Dexamethasone; Chorioretinitis
Cite this article
Bajaire-Gomez B, Sierra-Mejía H, Martinez-Roman S. Severe atypical resistant form of ocular toxoplasmosis using combined therapy: case report. Clin Surg J. 2022;3(4):1–9.
Copyright
© 2022 Boris Bajaire-Gomez. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).








