Case Presentation
A 59-year-old male presented with severe abdominal pain for the last three days in November 2021.
The pain was colicky around the umbilicus with a feeling of abdominal distention. It was associated with multiple episodes of vomiting and absolute constipation for the last 01 day. He had a normal appetite and normal bowel habits before this event. There were no episodes of such pain in the past. Past surgical history revealed that he was operated on for a (presumably) paraumbilical hernia about 15 years back. The record of which was not available. On examination, the patient had stable vitals. A generalized distention was evident upon inspection of the abdomen. Umbilicus was absent and was replaced by a midline scar about 15 cm long. Careful examination of the scar did not show any defects, swelling, or a visible/palpable cough impulse. There was, however, a diffuse swelling extending from the umbilicus towards the left hypochondrium. The swelling was ovoid, about 15 cm x 8 cm in size, with ill-defined margins. There was mild tenderness upon palpation of swelling, without any guarding or rebound tenderness.
An abdominal radiograph in an erect posture revealed multiple air-fluid levels, which were most central. The patient was diagnosed as a case of acute intestinal obstruction. He was admitted. The baseline laboratory workup was normal, including a normal leucocyte count. The patient was kept NPO, and standard conservative treatment of “drip-n-suck” was started.
Meanwhile, his CT scan was performed, which showed “loculated fluid along left hypochondrium, lower and mid abdomen causing mesenteric congestion’’. In the next 48 hours, the patient’s condition symptomatically improved with conservative management and giving rest to the bowel. The swelling, though decreased in size but was still palpable. The patient was started with a liquid to soft diet in a gradual fashion. He was discharged with the advice to come back if any symptoms recur. He remained well afterward. Upon a follow-up examination at a 04 weeks interval, the swelling was still palpable. This time a repeat CECT scan abdomen and pelvis was sought. Again, a loculated fluid collection area in the left hypochondrium region was found. This time it was reported that the fluid collection appears to be enclosing the small bowel loops, which were partially distended and drawn towards the center of the abdomen (Figure 1). No significant interval change was found in comparison with the previous CT scan.
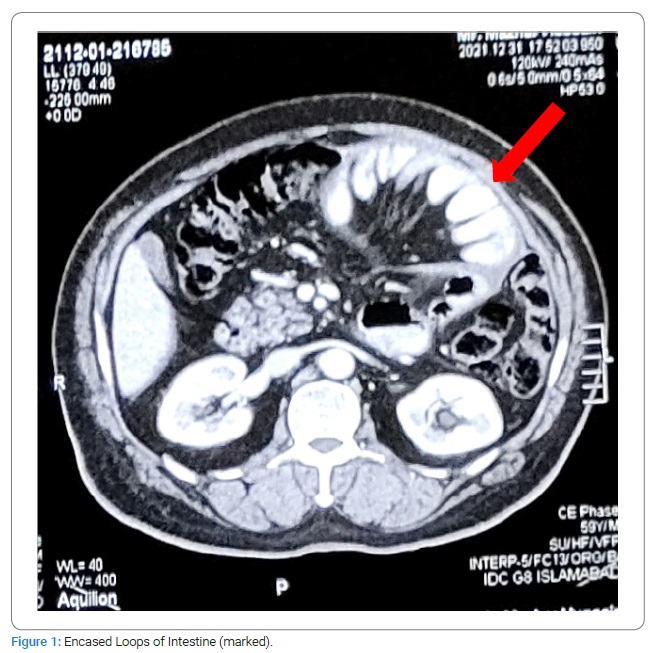
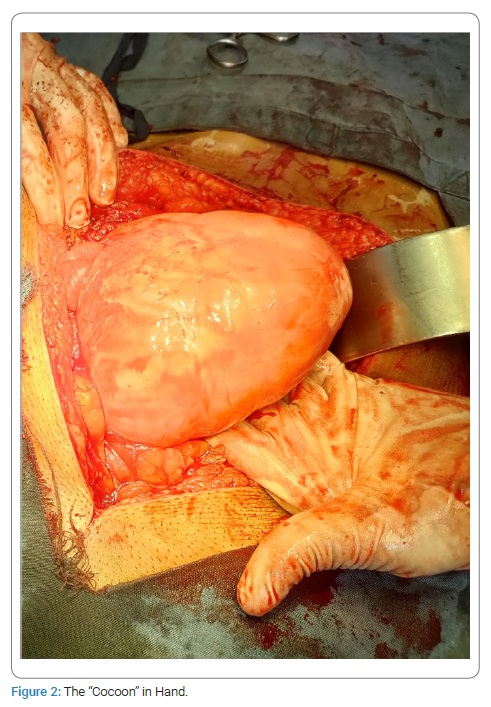
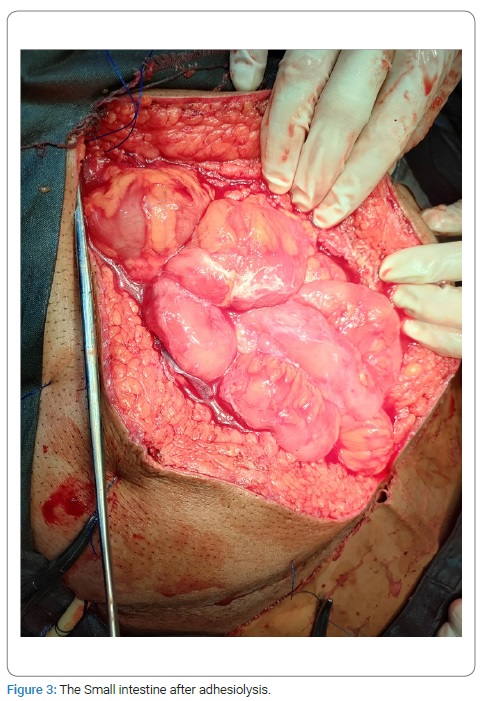
The persistence of the swelling conforming to matted gut loops and a consequent risk of a recurrent intestinal obstruction were discussed with the patient. An option was given to the patient for an elective exploration, to which he agreed. The patient was admitted, and an exploratory laparotomy was performed. Per-operatively the small intestine distal to DJ flexure was found wrapped in a fibrous silo (Figure 2). The author did not find any free small intestine; instead, a tough fibrous sheath was found, where jejunum was found entering it (soon after the DJ flexure), and the terminal ileum was exiting. Sac was opened, and the incorporated gut was found adherent to the inner surface of the sac as well as the bowel loops to each other. Luckily these adhesions were flimsy and were dissected easily (Figure 3). In the end, the gut was carefully observed for any damage, kink, or ooze; the cavity was washed with Normal Saline, and the abdomen was closed. Postoperatively the patient went well. NPO was broken after 12 hours. The patient was discharged after 48 hours. Histopathology of the sac wall revealed fibrous tissue without foreign body giant cells, granuloma, or malignancy.
Discussion
The condition Sclerosing Encapsulating Peritonitis (SEP) was first described nearly 100 years ago as ‘peritonitis Chronica fibrosa incapsulata’ [2–4]. The term abdominal cocoon syndrome was first coined by Foo et al. in 1978 [1]; however, not all cases of SEP fall under the category of abdominal cocoon syndrome.
SEP is considered to be instigated by an inflammation-inciting event. Depending on its etiology, it can be divided into primary (or idiopathic) or secondary. Primary or idiopathic SEP is termed abdominal cocoon syndrome. Several theories have been put forth in the past for explaining the primary SEP, including retrograde menstruation with a viral infection, retrograde peritonitis via the fallopian tubes, and any gynecological infection causing cell-mediated immunological tissue damage. These mechanisms were proposed keeping in mind the initial observation that most of these cases were reported in young females. However, these do not explain the mechanism in all of the patients, as around 75% of these patients are men, premenstrual females, or children [2,5,6]. Abdominal cocoon syndrome predominates in males with a ratio of around 1–2: 1 [7–10]. Another postulated theory is that it could be developmental and associated with vascular anomalies, omental hypoplasia or aplasia, absence of gastrocolic ligament, intestinal or colonic malrotation, visceral transposition, cryptorchidism, or hernia [2,6,8,11,12]. The exact cause is not well understood or validated despite these various theories.
Secondary SEP develops in response to some risk factors including, but not limited to, abdominopelvic infections (especially abdominal tuberculosis), history of abdominal surgery, long-term peritoneal dialysis, ventriculoperitoneal or peritoneo-venous shunts, malignant tumors, orthotopic liver transplant, and certain drug use including intraperitoneal chemotherapeutic drugs or povidone-iodine use, or B blockers (practolol or propranolol) [2,8,13–16]. Out of these, chronic peritoneal dialysis is the most common cause of secondary SEP [4].
SEP should be differentiated from Peritoneal Encapsulation (PE). It was initially described in 1868 by Cleland [17] as a developmental anomaly derived from the yolk sac peritoneum during the early stages of fetal development resulting in an accessory peritoneal membrane which is found between the mesocolon and the omentum, and most of the small intestine lies posterior to it [2]. In contrast to the abdominal cocoon, the PE membrane is usually thin; patients are asymptomatic and rarely present with intestinal obstruction [18]. These are incidentally found during laparotomy for other indications [2,13,19].
Abdominal cocoon syndrome can be classified into three categories depending on the extent of encasement of the cocoon. In type I, part of the small bowel is enveloped, whereas in type II the entire small bowel is encased. In type III, the membrane covers other organs besides the small bowel, including the appendix, cecum, large intestine, ovary, liver, or stomach [20]. In our patient, it was type II because the entire small bowel was encased without the involvement of any other organs in the abdomen.
Patients usually present with vague abdominal symptoms, and hence delay in diagnosis is seen. Initially, patients report fever, abdominal pain, and altered bowel habits [2,4,21]. As the disease progresses and the membrane develops over several years, it may develop into an intestinal obstruction. However, it has also been reported to develop rapidly within 12 weeks from the onset of symptoms [2,20,22]. Rarely may patients present with life-threatening complications, including enterocutaneous fistula, bowel perforation, or malnutrition [2]. Machado et al. reported 118 cases of abdominal cocoon syndrome and found abdominal pain as the most common manifestation (72%), followed by abdominal distention (44.9%) and an abdominal mass (30.5%) [20]. In another case series of 24 patients, Wei et al. reported complete or partial obstruction in 88% of patients with abdominal cocoon syndrome, and an abdominal mass was felt in 54% of the patients [6].
Correct diagnosis before surgery is challenging, and it has been reported that the rate of misdiagnosis can be as high as 95% of the time [8]. Laboratory tests are usually non-specific, and so the diagnosis is largely based on clinical suspicion and radiology findings. Starting with an x-ray, a plain abdominal film is again not specific, and if the patient has advanced to the obstructive stage, then it would show dilated small bowel loops on supine film and air-fluid levels on erect film, and occasionally bowel wall and peritoneal calcification [2,4,20,21,23]. A barium study may show bowel loops conglomerated in the center of the abdomen, which might point to the diagnosis. However, as most of these patients present with acute or subacute intestinal obstruction, barium studies might not be feasible for these patients. Ultrasound of the abdomen may show dilated bowel segments encased by a dense fibrous membrane or abdominopelvic ascites and a thickened peritoneum. However, ultrasound is highly user-dependent and prone to missing crucial findings depending on the performer’s expertise [2,4,24].
The diagnostic study of choice is a Contrast-Enhanced CT scan (CECT) of the abdomen and pelvis. Several findings have been described in conjunction with SEP on a CT scan. First, the peritoneum is usually thickened with marked continuous enhancement. Even though there is no agreed-upon cut-off value for peritoneal thickness to be suggestive of SEP, a thickness of more than 2 mm has been proposed [19]. Other findings on the CT scan include clumping of intestinal loops together, the cauliflower sign (where there is a broad ‘head’ formed by the distended bowel loops and a narrow mesenteric ‘apex’), and the bottle gourd sign (dilation of the 2nd and 3rd part of the duodenum and encasement of the distal duodenum and jejunal loops) [19,25].
Even though radiological investigations can point to a diagnosis of SEP, confirmation is usually done intraoperatively once the fibro-collagenous membrane is encountered, and postoperatively, with the histopathological examination of the excised membrane, which would show a complete loss of the mesothelium associated with significant interstitial thickening composed of fibroblasts and collagen deposition within the peritoneal membrane [26].
With regards to the treatment options, the authors argue that in patients with mild abdominal symptoms, conservative management should be employed for as long as possible [27,28]. This includes bowel rest, gastric decompression, and parenteral nutrition. In addition, decreased oral intake and malnutrition are common in this population because of repeated bouts of intestinal obstruction, and poor nutritional status has been identified as an independent factor in postoperative complications [21]. Therefore, improving a patient’s nutrition is a key factor in the success of either medical or surgical treatment [2].
If the patient fails conservative management, certain anti-inflammatory drugs may be used, including steroids, tamoxifen, colchicine, azathioprine, or mycophenolate mofetil [4,27,29–31]. These drugs are efficacious in secondary SEP, and few studies have shown their utility in primary SEP or cocoon syndrome [27,32]. Some authors recommend giving a trial of these medications in type II or III abdominal cocoon syndrome, in whom adequate excision and adhesiolysis could not be achieved, or in patients with recurrent symptoms even postoperatively [2].
The most effective treatment for idiopathic cocoon syndrome in patients with severe obstructive symptoms remains surgical excision of the membrane and adhesiolysis [21,33,34]. The risk of recurrence is negligible if the membrane is completely excised [10,21,35]. There is not a lot of strong data in favor of instilling an antiadhesive substance in-between the intestinal loops before closing up the abdomen to decrease the risk of postoperative adhesive bowel obstruction [6,36]. In rare cases where patients present with bowel perforation, depending on the degree of contamination and condition of the adjacent bowel, patients can undergo resection and primary anastomosis or go towards a diverting ostomy.
The most common postoperative complication is early postoperative small bowel obstruction (EPSBO). It usually develops within 30 days postoperatively in patients undergoing extensive bowel manipulation and adhesiolysis, resulting in intestinal edema and prolonged operative times [2,21,37]. However, it is mostly temporary and resolved with conservative management. Our patient already had a previous episode of small bowel obstruction, and a mass was still palpable, so the decision was made to proceed with surgery, especially because a preoperative diagnosis had also been established and the patient was in a healthy state.
Looking at the natural history of the disease, these patients usually suffer from recurrent episodes of intestinal obstruction before finally undergoing surgery, which begs the question of the utility and efficacy of the early surgical intervention in patients who have once developed obstructive symptoms, even if it has been relieved with conservative measures. However, further studies need to look at the ideal timing of surgical intervention in these patients, which might be difficult logistically due to the rarity of the disease process.
Cite this article
Hameed FM, Ahmed SM. Acute intestinal Obstruction with a palpable mass: ‘Abdominal Cocoon Syndrome’. Clin Surg J. 2022;3(4):1–6.



