Abstract
Objectives: The study aimed to describe the clinical, microbiological, and therapeutic characteristics of septic arthritis after Anterior Cruciate Ligament Surgery (ACLR).
Methods: A nine-year retro-prospective study was conducted in two French centers. All the patients presenting knee arthritis symptoms were included. We collected epidemiologic, clinical, bacteriological, therapeutic, and outcome information.
Results: Among 12,650 patients, we identified 95 cases of septic arthritis after ACLR. The mean age was 32 years, and 87 patients were male. All the patients showed local signs of inflammation; 67 presented fever. The infection rate was different according to the type of ACLR. The median delay between ligament reconstruction and surgical lavage for infection was 20 days, and 90 deep samples were positive in culture. The main bacterial species found were coagulase-negative staphylococci, Staphylococcus aureus, Cutibacterium acnes (C. acnes). Arthroscopic surgery was performed for 94 patients, with at least one procedure for twenty-one. Seventy-one patients were treated with the combination levofloxacin-rifampicin for six weeks mean duration. The outcome was favorable for 91 patients.
Conclusion: Septic arthritis remains a rare early complication after ACLR. Coagulase-negative staphylococci are the main bacteria involved with low rates of antibiotics resistances. Treatment consists of a six-weeks levofloxacin-rifampicin association and surgical treatment leading to a favorable outcome.
Material and Methods
We conducted a nine-year (January, 2011–December, 2019) retrospective (from 2011 to 2013). Then a prospective study in two major orthopedic centers itn the same urban area (southwest of France) where all patients > 18-year-old with Anterior Cruciate Ligament (ACL) infections were referred to the same infectious diseases team.
All patients managed in the orthopedic unit of these hospitals with knee arthritis following ACLR were included. The outcome of the patient was based on consultations with at least 1-year follow-up. A clinical evaluation was performed during the consultation. A favorable outcome was established on a good clinical recovery, no sign of inflammation, and satisfying joint mobility. No standardized functional score was used during the follow-up in this study.
Diagnosing ACL septic arthritis was based on clinical, biological, and microbiological arguments. Suggestive clinical signs of arthritis were those classically described, including fever and local signs (knee pain, swelling, warmth, and restricted movement).
CRP was measured as soon as the infection was suspected and used as a biological marker for follow-up and clinical evaluation. To complete this investigation, we checked the reason for subsequent hospitalization for all patients who were readmitted during the year following ACLR. We selected epidemiologic, clinical, bacteriological, and therapeutic data from the patient schedule. In addition, the subsequent surgeon and infectious disease specialist counseling letters analyzed information regarding short and mid-term follow-ups.
The institutional review board approved the study design. All the patients received full information about the procedure.
Results
Among 12,650 patients who underwent ACLR during the study period, we identified 95 cases of knee arthritis (incidence rate = 0.75%). Mean age was 32 years, 87 patients were male (sex ratio = 10, 9). All patients received a first or second-generation cephalosporin (cefazolin or cefuroxime) preoperatively intravenously as antimicrobial prophylaxis. The most common procedure used for ACLR was an allograft using the hamstring (semitendinosus and gracile) tendon (for 87.2% of the patients), then an allograft using the patellar tendon (for 12.8% of the patients). Infection rate (IR) was different according to the surgical technique: slightly more than twice higher for the hamstring allograft (IR = 0.80%) than for the patellar allograft (IR = 0.37%) (p < 0.001). The mean time from suspicion of infection to its management was 4.5 days. The median time from ACLR to surgical revision for infection was 20 days. All patients had clinical signs of joint inflammation (pain, swelling, tenderness), and almost 71% of patients had a fever. A high CRP value (> 20 mg/L) was found in all cases with a mean value of 148 mg/L, and 81% of patients had a CRP higher than 100 mg/L.
Culture from intraoperative samples was sterile for five patients who had received antibiotics before the intervention. The culture was polymicrobial in 19% of cases. A total of 111 bacteria were identified and are shown in (Table).
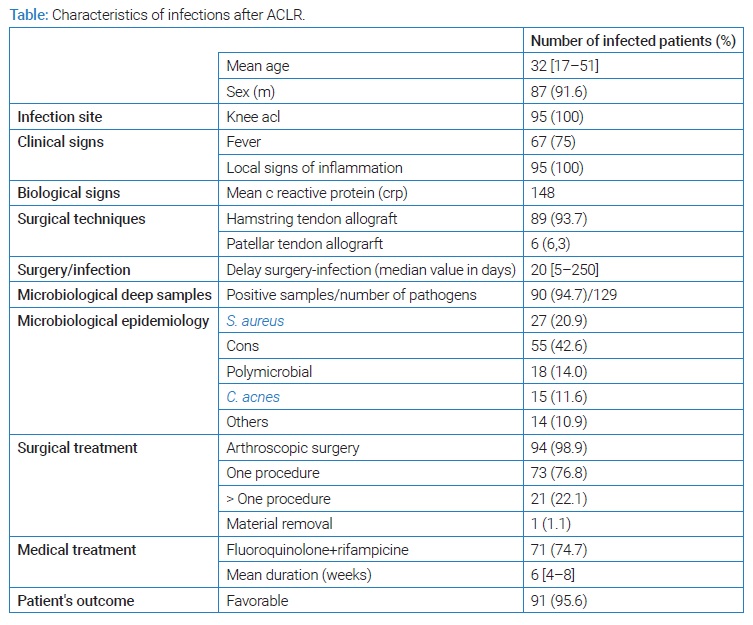
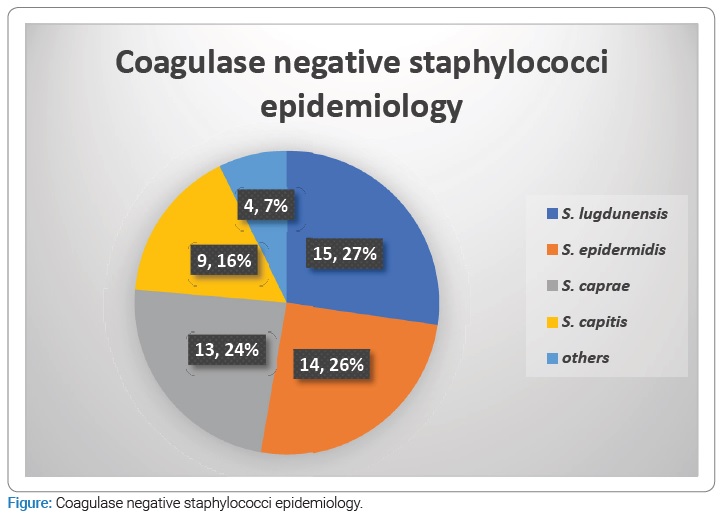
Staphylococci represented almost three fourth (73.9%) of all bacteria, S. aureus accounting for 24.3% and coagulase-negative staphylococci (CoNS) for nearly half (49.5%) of all isolates. Among CoNS, the most frequent species were S. lugdunensis, accounting for 13.5% of all bacteria. The second most frequent CoNS was S. epidermidis (12.6%). The repartition of CoNS is detailed in (Figure).
Fourteen (17%) staphylococci strains were resistant to oxacillin: one S. aureus and 13 (23.6%) CoNS. All S. lugdunensis strains were susceptible to oxacillin. No staphylococcus strain was resistant to vancomycin, daptomycin, or linezolid, and only two (2.4%) were resistant to quinolones, none during the last seven years. Only two strains (2.4%) were resistant to rifampicin.
Cutibacterium acnes was found in 15 (15.8%) cases and accounted for 13.5% of all pathogens; other pathogens (Gram-negative bacilli, streptococci, and enterococci) accounted for 12.6%. No fungal infection was identified.
All patients but one had at least one arthroscopic lavage. Seventy-three (76.8%) patients underwent one lavage, 16 (16.8%) required two lavages and 5 (5.3%) patients three lavages. One patient had a removal of all material right away. Before we standardized the empirical antibiotic treatment in 2013, various antibiotics were used by surgeons, including IV anti-staphylococcal agents (vancomycin for one-third of patients and cloxacillin for nearly one-third of patients). From 2013 to 2017, standardized empirical postoperative antibiotics included vancomycin and piperacillin-tazobactam or ceftriaxone. Since 2017, we have switched to a probabilistic combination of daptomycin and ceftriaxone. As almost all staphylococci were susceptible to rifampicin and quinolones, 74.7% of patients received an oral combination of levofloxacin and rifampin as second-line treatment, 5 to 6 days after the arthroscopic lavage. All strains of enterobacteriaceae and C. acnes were also susceptible to quinolones.
The mean duration of treatment was six weeks (4 to 8 weeks depending on the improvement time). The duration of follow-up was at least 24 months for all patients. The cure rate was 95.6%, with the occurrence of infection relapse in 4 patients.
Discussion
Infections after ACLR remain rare diseases, with 95 cases on 12,650 patients reported over a period of nine years. ACL infections occur early after surgery. Staphylococci are the main bacteria involved with low rates of antibiotics resistances. These infections are managed by a combination of medical and surgical treatment with a favorable outcome.
The incidence rate of infection following ACLR is quite low, usually lower than 1% [3,4,8–13]. We found this rate in our series, with a rate decreasing in recent years. Infection’s rate was reported from 0.32% to 1.8% [1,2,14]. This variable rate has been described according to the technique for ACLR, with a higher incidence rate (two to eightfold higher) for hamstring tendon allograft as compared to patella tendon allograft [3,4,12,14]. We also found such a difference in our series since the infection rate was 2.16 times higher for hamstring tendon allograft.
As ACLR is often used for young adults practicing sports, it is not surprising to have a mean age of 32 years. However, an even lower age was found in other series [3,12,14]. The predominance of male patients could be explained by practicing sports such as rugby which is popular in this area and more frequently played by men.
The clinical presentation is typically the one of acute septic arthritis with local inflammatory signs and fever. As expected, CRP results were high, according to previous reports showing it as a useful biological marker in acute bone and joint infections. In a study including 122 patients, the mean CRP value in patients with septic joints was 101.9 mg/L, significantly higher than in the non-infection group (P < 0.01). A CRP value of 41 mg/L was the optimal threshold to predict infection, with a sensitivity of 94.1% and specificity of 97.6% [15].
Epidemiology: Regarding bacterial epidemiology, staphylococci are the main pathogens found in ACL infections [5,16]. We reported 73.9% of staphylococci involved in 95 cases which is more than the Kursumovic et al. study (51.7%) but in accordance with Kim et al. results (75.6%) [6,17].
As shown in other studies, bacteria species most frequently involved are CoNS accounting for 49.5% of all isolates [4,6,7,18]. Among CoNS, S. epidermidis is particularly frequent [19–21], but we found other species often involved, such as S. lugdunensis, S. caprae, and S. capitis. One case of S. capitis ACL infection was described by O’Neill in 2013, and Wang et al. reported rare cases of ACL infections due to S. hominis and S. haemolyticus [22,23]. Our study shows that S. lugdunensis was the most frequent CoNS species before S. epidermidis (12.6%). Few reports have already described rare cases of S. lugdunensis ACL infections [24,25], but this CoNS, which pathogenic power is quite like S. aureus is described in bone and joint infections [26].
S. aureus is also reported as a frequent pathogen which is concordant with the 20 cases found in this study [4,6,7,18]. In addition, some studies described streptococci and E. faecalis as pathogens involved in ACL infections [4,6,11]. However, other species were less described like anaerobes (Peptostreptococcus sp.), enterobacteriaceae (E. coli, Klebsiella sp.), Pseudomonas sp. or Nocardia sp., as we found in our study with 12.6% of ACL infections due to Gram-negative bacilli, streptococci, enterococci and 19% of polymicrobial infections [9,11,17].
C. acnes infections were also described as accounting for up to 12% of all ACL infections [5,27,28], which is in accordance with our results (13.5%). In Wang et al. study, 92% of C. acnes ACL infections may be defined as acute infections occurring less than one month after surgery [15].
Physiopathology: Some reports showed that biofilms are frequently present in bone and joint infections, and C. acnes are often involved in biofilm formation [29,30].
Recently, Hiller et al. showed bacterial colonization of grafts in ACL even if no clinical signs of infection were reported [29]. The colonization rate of grafts may amount to 23% and be a factor involved in graft rupture [31,32]. Molecular methods like mass spectrometry were used to detect bacteria on graft samples, and Hiller et al. reported positive results on most samples [29]. Bacteria localized in the biofilm are not detected and cannot be found on cultures without the appearance of clinical signs [31], and colonization was significantly more detected on torn graft tissue compared to a torn native ligament or hamstring tendon autograft. Some studies reported the presence of biofilm on monofilament material braided sutures [32–36] as reported in knee infections.
Resistances: Methicillin resistance is not rare among CoNS strains, particularly those involved in bone and joint infections, with a higher rate for S. epidermidis species [26]. Regarding this species specifically, we found 50% of methicillin resistance which is in line with the Kunovac et al. study showing 45% of the strains resistant [7]. As we also reported, S. lugdunensis described in previous reports showed susceptibility to methicillin or cefazolin for all the strains [26]. Regarding S. aureus antibiotic susceptibility, we found 5% of the strains methicillin-resistant as the few studies that have described less than 10% [7,20,37]. Our study found no resistance to vancomycin, daptomycin, and linezolid for staphylococci strains, in line with previous reports [19,20,37]. Resistances of staphylococci to other antibiotics like fluoroquinolones or rifampicin were rarely specified in previous studies, but we found only 2.4% of staphylococci resistant to fluoroquinolones and rifampicin. E. faecalis are bacteria commonly susceptible to amoxicillin, and no resistance has been described in ACL infections [5,16].
Antibiotic prophylaxis: Antibiotic prophylaxis is recommended prior to surgery [38,39]. First-generation cephalosporin is usually administered shortly before surgery or clindamycin in case of allergy [7,11]. Some authors reported the use of prolonged antibiotic prophylaxis to reduce the risk of infection [40]. Antibiotic prophylaxis may include using a vancomycin wrap to pretreat the hamstring graft. This technic was first described in 2012, but it is still not clearly demonstrated if it significantly reduces rates of ACL postoperative infections compared to standard antibiotic prophylaxis [41].
Surgical treatment: As recommended for treating such arthritis, an articular lavage has been applied to all patients except one for whom the surgeon removed all the material [38,39]. In most cases, one arthroscopic lavage was sufficient to obtain a favorable outcome, but in 22.3% of patients who had the first lavage, at least one additional lavage was necessary to obtain clinical and biological improvement. A favorable outcome was observed only after the third wash for five patients. In the review of Kim et al., an average of 1.92 procedures was found to be necessary to eradicate infection [11]. Surgeons should not hesitate to perform one or more additional arthroscopic lavage if fever or pain persists or even increases in the 72 hours following arthroscopic lavage and appropriate antibiotic therapy.
Antibiotic empirical therapy and oral antimicrobial treatment: ACL infections antibiotic treatment consists first of empirical intravenous antibiotic therapy. Treatment is then adapted according to antibiotic susceptibilities of pathogens found in culture. In some cases, although patients presented clinical symptoms of acute ACL infection, microbiological diagnosis remains negative. The rate of negative cultures varies from 21.9% to 58.7% of the cases [7], but we found only 5.3% of negative cultures, and this concerned patients under antibiotic therapy before surgery.
According to national and international recommendations, an empiric therapy associating a third-generation cephalosporin with daptomycin or vancomycin is recommended [38,39]. In addition, the epidemiology of ACL infections shows a great majority of staphylococci and methicillin resistance is frequently described in CoNS infections. Therefore, although GNB is not often involved, it is relevant to propose a third-generation cephalosporin active on these bacteria in association with daptomycin or vancomycin.
Antibiotic treatment is then adapted to microbiological results, including IV antibiotics followed by oral treatment. The choice of antibiotics must consider bone diffusion, action against biofilm, and the prevention of small colony variants observed in bone and joint infections [38,39].
The oral treatment is prescribed according to antibiotic susceptibilities and should include rifampicin associated with another drug. Rifampicin is very active on intracellular bacteria, and previous studies reported its efficiency against biofilm [38,39].
The best oral companions of rifampicin are fluoroquinolones, including levofloxacin, ofloxacin, or ciprofloxacin. These antibiotics are efficient in staphylococci, streptococci, and enterobacterial ACL infections. Moreover, this combination showed the inhibition of biofilm formation, a good penetration associated with a bactericidal activity into the biofilm [38,39].
Other agents such as quinolones may be used in case of antibiotic resistance, allergy, or intolerances. Cotrimoxazole, tetracyclines, and clindamycin are effective drugs in bone and joint infections [38,39].
Antimicrobial treatment duration was six weeks for most patients, agreeing with French guidelines and previous reports on the management of septic arthritis [7,28]. However, the duration may be shorter in some studies reporting a minimum of four weeks of antibiotic treatment [19,42]. In addition, continuous parenteral treatment may be pursued until CRP normalization before switching to oral treatment, but the criteria leading this choice depend on the center’s practices [7,23].
Our high cure rate (> 95%) could be explained by early and multidisciplinary management combining arthroscopic lavage, repeated if necessary, and an antibiotic combination including rifampicin. The favorable outcome of most patients was also reported in previous studies but almost in case reports or small case series with less than two years of follow-up [6,7,19].
The aim of the present study was not to evaluate the long-term functional prognosis. However, surgeons will set up a prolonged follow-up to evaluate it since some authors have suggested the existence of residual complications such as arthrofibrosis or loss of joint space [2,42].
Cite this article
Lourtet-Hascoët J, Javois C, Potel JF, Merouani M, Bouige A, Giordano G, et al. Septic arthritis after anterior cruciate ligament reconstruction: Microbiological epidemiology, treatment, and outcome A 95 case-series among 12,650 patients. Clin Surg J. 2022;3(3):1–7.


