Effect of COVID-19 Infection in Pregnancy and Perinatal Outcome. A Case Series Study
* Humaira Saeed Malik;
Huma Parveen Khan;
Issam bin Ali;
Abdul Kadir Al-juhani;
Ahmed Rezan Seida;
Haifa Sindi;
Essam Mohammad Abbas;
-
* Humaira Saeed Malik: Department of Obstetrics and Gynecology, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
Huma Parveen Khan: Department of Obstetrics and Gynecology, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
Issam bin Ali: Department of Obstetrics and Gynecology, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
Abdul Kadir Al-juhani: Department of Pediatrics, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
Ahmed Rezan Seida: Department of Pediatrics, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
Haifa Sindi: Department of Pediatrics, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
Essam Mohammad Abbas: Department of Pediatrics, Royal Commission Medical Center, Yanbu, Saudi Arabia.
-
May 09, 2022 |
-
Volume: 3 |
-
Issue: 2 |
-
Views: 1406 |
-
Downloads: 1523 |
Abstract
The objective of this case series study was to evaluate the obstetric, perinatal, and neonatal outcomes of pregnant women who tested positive for the COVID-19 (Coronavirus) infection. All pregnant patients (n = 21) who tested positive for the COVID-19 infection using RT-PCR at the Department of Obstetrics and Gynecology, Royal Commission Medical Center Yanbu, Saudi Arabia from July 14, 2020, to November 14, 2020, were included in the study. Data regarding their demographics, clinical presentation, maternal, perinatal, and neonatal outcomes were collected. Most (n = 18, 85%) of the patients were multipara in the third trimester of pregnancy (n = 20, 95%). Out of the 21 patients eleven (52.3%) women had mild symptoms while ten (47.6%) were asymptomatic. The symptoms reported by patients were cough (52.3%), fever (28.5%), shortness of breath (9.5%), and diarrhea (9.5%). The adverse outcomes associated with the pregnancies included: one (4.7%) missed miscarriage, three (14.2%) preterm deliveries, three (14.2%) fetal distress, one (4.7%) abruption placentae, and one (4.7%) intrauterine fetal death. Among the 20 delivered women (including two with twin pregnancies) 30% (n = 6) had a vaginal delivery and 70% (n = 14) underwent cesarean section for obstetric indications. The mean birth weight was 3019 grams ± 577.4 grams and a low APGAR score was not observed. The presence of SARS-CoV-2(Severe acute Respiratory Syndrome coronavirus 2) was detected in three neonates (16.6%), including a set of twins out of 18 neonates. Ten (47.6%) neonates develop respiratory distress. There were no maternal or neonatal deaths. In conclusion, this study found that the COVID-19 infection caused mild symptoms amongst pregnant women in Yanbu, Saudi Arabia, and maternal to neonate transmission was low.
Introduction
The coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), which is transmissible through respiratory droplets [1,2]. The spread of the virus has caused many health concerns [3,4]. Clinical features of the illness are variable, ranging from asymptomatic infection to mild symptoms to severe symptoms, which can result in pneumonia and respiratory failure [2,5,6]. Pregnant women are at greater risk for developing a severe illness compared to non-pregnant women [7]. This was demonstrated by pregnant women who tested positive for COVID-19 having an increased rate of admission to the intensive care unit, need for supplemental oxygen, ventilation, and mortality [8]. This may be because pregnancy is characterized by physiological changes including increased heart rate and oxygen consumption, decreased lung capacity, a shift away from cell-mediated immunity, and increased risk for thromboembolic disease. To better understand the effect of the COVID-19 infection on pregnant women in Yanbu, Saudi Arabia, this study aimed to investigate the maternal, perinatal, and neonatal outcomes in pregnant patients with COVID-19 infection.
Material and Methods
This case series study was conducted on pregnant women with confirmed COVID-19 infection, admitted to the Department of Obstetrics and Gynecology of Royal Commission Medical Center, Yanbu, Saudi Arabia, during the first wave of COVID-19, from June 14, 2020, to November 14, 2020. A precise diagnosis of COVID-19 infection was made by the detection of SARS-CoV-2 (COVID-19) positivity on RT-PCR analysis of nasopharyngeal and oropharyngeal specimens collected from the women. The inclusion criteria were all pregnant patients in whom COVID-19 infection was laboratory-confirmed, whereas the exclusion criteria were pregnancies with negative RT-PCR results for SARS-CoV-2. Informed consent was obtained from all patients and the study protocol was approved by the hospital ethics committee. Data Collection: Data was collected and recorded on the structured proforma. The following data were collected: demographic (age, parity), gestational age at admission and at delivery, detailed history of presenting complaints, medical comorbidities, and any symptoms of pneumonia-like fever, cough, shortness of breath, and diarrhea. Mode of delivery (vaginal, including instrumental, or cesarean) recorded. Maternal, neonatal outcomes and perinatal outcomes (fetal outcomes including birth weight and stillbirth) were also noted. Stillbirth is defined as fetal death after 24 weeks of gestation. The neonatal period begins from birth and ends 28 days after birth. Neonatal outcomes were assessed using the APGAR score at 1 min and 5 min of life. Low APGAR scores are considered lower than 7 at 1 minute and 5 minutes after birth. Other neonatal parameters recorded were neonatal symptoms, admission into neonatal ICU, neonatal death, and presence of COVID-19 infection in the neonate. The latter was done to determine whether maternal to fetal transmission of SARS-CoV-2 occurred and was determined through neonatal nasopharyngeal swabs at birth (collected after cleaning the baby) and at 24 hours –48 hours of age. The neonate was considered to have COVID-19 infection if the RT-PCR from nasopharyngeal/oropharyngeal swab from the infant or blood from neonate/umbilical cord or amniotic fluid or tissue sample from the fetal side of the placenta tested positive for SARS-CoV-2. Safety Protocol: All precautions and Personal Protective Equipment (PPE) as per hospital policy were utilized by hospital staff to limit infection transmission for suspected and confirmed cases until the result of the swab test. Statistical Analysis: Percentages and mean/median values are calculated to describe categorical and continuous variables, respectively. SPSS v23 was used for statistical analysis.
Results
A total of 21 pregnant women were tested to be COVID-19 positive. The demographics of these 21 patients are shown in (Table 1). The mean ± SD for age (years) was 32 ± 6.4. A majority (85.7%) of the patients had previous pregnancies and most (n = 11) of them had singleton pregnancies while two patients had twin pregnancies. Gestational age at admission ranged from 11 weeks + 6 weeks to 40 weeks and the mean ± SD gestational age (in weeks) was 36.47 ± 5.97 (Table 1).
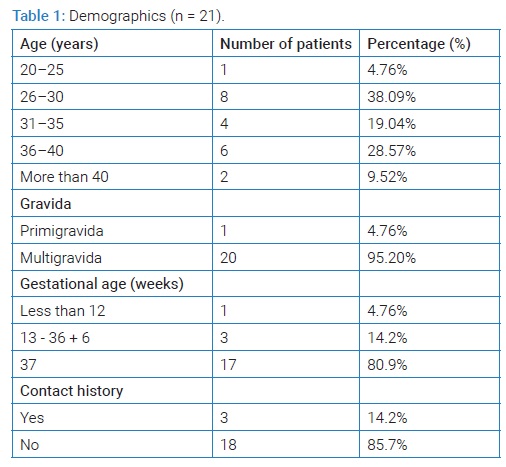
The clinical presentation of the patients is summarized in (Table 2). Out of these 21 patients, eleven (52.3%) women were symptomatic with mild symptoms while 10 (47.6%) were asymptomatic. The patients had the following comorbidities: hypothyroidism (n = 2), asthma (n = 2), gestational diabetes (n = 1) and gestational thrombocytopenia (n = 1). All these comorbidities were well controlled. Out of 21 women, one was admitted the in the first trimester at 11 weeks 6 days with a missed miscarriage (Table 3). She presented with bleeding prevaginal and was managed by suction evacuation of the uterus. Twenty (95%) women were admitted in the third trimester out of which 30% (n = 6) had vaginal delivery and 70% (n = 14) underwent cesarean section for obstetric indications. Among these 20 women three patients had preterm deliveries beyond 36 weeks (Table 3), three had fetal distress, one developed abruptio placentae, and one had intrauterine fetal death. The patient who had stillbirth was 30-year-old multipara with no significant comorbid conditions. She presented at gestational age 40 weeks and 1 day, with cough, diarrhea, and nausea. She was offered induction of labor which she refused later she delivered a 3300-gram stillborn infant by vaginal delivery. Her placental histopathology was normal. None of the patients developed severe pneumonia, required mechanical ventilation, intensive care unit (ICU) admission, or died and all patients recovered. The fetal and neonatal outcome is described in (Table 3).
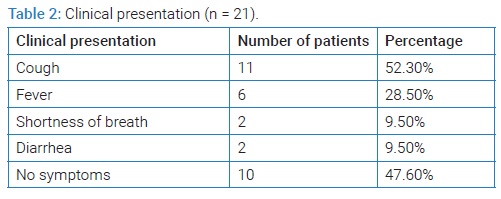
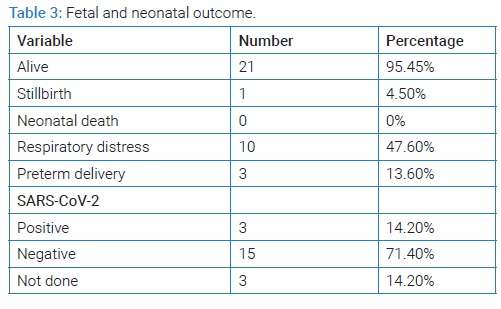
Among the 21 alive neonates, low birth weight was recorded in two (9.5%). The mean birth weight ± SD (grams) was 3019 ± 577.4 and APGAR scores at 1 and 5 minutes ranged from 7 to 8 and 8 to 9 respectively. The presence of SARS-CoV-2 was tested in 18 out of 21 neonatal throat swabs, which were positive in three (16.6%), including two sets of twins. The positive neonates were delivered by cesarean section and had mild respiratory symptoms. No neonatal death was recorded.
Discussion
In Yanbu, Saudi Arabia, during the peak of the COVID-19 (Coronavirus) pandemic, many pregnant women attended our hospital which was a designated hospital for COVID-19 positive patients, with symptoms of COVID-19 infection, history of contact with COVID-19 infected patients, obstetrical indications, and childbirth. RT-PCR was done in all symptomatic patients and those who were being admitted for surgical intervention. This study includes an analysis of 21 pregnant women with COVID-19 infection who was admitted to our hospital. The mean age of the patients was 32 ± 6.4 ranging between 26 years to 46 years. This is consistent with the findings of other researchers [9,10,11] Majority (95.8%) of these patients were multigravida while only one was primigravida, which is similar to findings of other studies where 80% were multipara [10]. Most (n = 11) of them had singleton pregnancies while two patients had twin pregnancies. The vast majority of women were in the third trimester with the mean gestational age at admission 36.47 weeks’ ± 5.97 weeks. These findings are comparable to other studies [9,11,12]. Out of 20 women, three (15%) women had a preterm birth, and the cause of preterm birth was not related to COVID-19 pneumonia or obstetrical reasons. In a systematic review, preterm birth was three times more common in individuals with COVID-19 than among those uninfected, with rates of 16% vs. 6%, respectively. However, whether this difference is due to direct effects of infection or maternal illness or is iatrogenic is unknown [13]. Many researchers reported that the most common reasons behind preterm births were due to worsening maternal COVID-19 or obstetric complications unrelated to COVID-19 disease [14]. It may be associated with a storm of pro-inflammatory cytokines accompanying the acute course of SARS-CoV-2 infection [15]. Evidence with regards to preterm birth and perinatal mortality is conflicting, but these risks are generally higher only in symptomatic, hospitalized women [13]. The predominant symptom noted was cough in eleven (52.3%) patients followed by fever in six (28.5%) patients.
These findings were also reported by other researchers [7,16]. Other symptoms noted were shortness of breath and gastrointestinal symptoms. In contrast, a systemic review of 104 cases documented fever, cough, and dyspnea in 58.6%, 30.7%, and 14.4% respectively [17]. Our study showed that 10 (47.6%) women were asymptomatic. This is, in contrast, to a study where asymptomatic rates were higher [18]. All those symptomatic pregnant women had mild symptoms. They were managed conservatively, and they had a quick recovery, and this is consistent with other studies [16,19,20]. None of the patients developed severe pneumonia or needed Intensive Care Unit (ICU) admission or died. This is also observed in other case series [9,16]. In contrast, some studies reported a higher incidence of serious pneumonia [21,20]. Maternal deaths in women infected with COVID-19 during pregnancy were also reported [18]. In this case series, one woman had a first-trimester miscarriage. She was managed by suction evacuation of the uterus. This is also observed in another study [22], but to date, there is no evidence that SARS-CoV-2 infection during the first trimester of pregnancy increases the risk of early pregnancy loss [23].
Intrauterine fetal death at 40 weeks +1 day was observed in one woman. She had only mild symptoms of dry cough and diarrhea, so the cause of stillbirth was unexplained. In a study by Liu et al, 2020 [20], fetal death at 34 weeks was reported but the cause of fetal death was speculated to be severe maternal pneumonia and multiple organ failure rather than viral infection of the fetus. In a recent report from 16 public health jurisdictions in the US on pregnant patients with laboratory-confirmed SARS-CoV-2 infection, stillbirth occurred in 0.4% of cases, which is not higher than the background risk [24]. In contrast in an analysis from the UK, the rate of stillbirths was two to three times higher among pregnant individuals during vs before the pandemic, with rates of 9.3 vs 2.4/1000 births, respectively, although whether the increase is related to SARS-CoV-2 infection or other pandemic-related factors is unknown [25]. The medical comorbidities and complications noted in this case series were gestational diabetes, asthma, hypothyroidism fetal distress, abruption placenta, and prelabour rupture of membranes which is also reported by systemic reviews by other researchers [16,17]. Most (70%) women had cesarean delivery while 30% had a vaginal delivery. Additionally, three women were induced. The indication of cesarean section was unrelated to the COVID-19 infection and was due to obstetric indications. This is lower than reported in other studies [9,20], but is higher than Italian study where most (57%) women delivered vaginally [26] and a national cohort study reported by Knight et al. 2020 [27]. where the cesarean rate in pregnant women with COVID-19 was about 59%. The test for SARS-CoV-2 was performed on 18 neonatal throat swabs out of a total of 21 alive neonates. Three (16.6%) neonates were positive. All three neonates were delivered by elective cesarean section. These results are consistent with findings by Yang Z, 2020 [28] who reported that two neonates out of 84 live births tested positive for RT-PCR at 36 hours and three days of age but also stated that postnatal infection was possible. This is in contrast to other publications that observed no positive results in all neonates who were tested [18,20]. Intrapartum or early postnatal infection could occur through the exposure of the delivering neonate to infected maternal blood or secretions. Both may be considered examples of “vertical” transmission. The mean birth weight was 3019 grams ± 577.4 grams, similar to a study reported in Pakistan [11]. Two (9.5%) neonates had a low birth weight of less than 2500 grams. On the contrary, a systemic review demonstrated a higher incidence of low birth weight. All live births had an APGAR score of more than 7 at 5 min which was also reported by Chen H, 2020 [9] whereas some studies reported lower APGAR scores [26,27,28]. Respiratory distress was the most common symptom noted in 10 neonates (47.6%) including COVID-19 positive neonates. Other studies also show the variable incidence of Respiratory Distress Syndrome (RDS) [25,26,27]. ICU admission was done in 18 neonates for observation and isolation. This was also observed in other studies [10,16]. No neonatal death was recorded in this study. The literature demonstrated mixed results in regards to neonatal death, with some reporting none [9,20], while others reporting some neonatal death [16,27].
There are many limitations to our study. Firstly, the sample size is small and is limited to one city and one medical center, so the results cannot be generalized. To improve the study and better understand the effect of COVID-19 on pregnancy and perinatal outcomes, a larger and more representative sample size is required. The second limitation is that there was no universal testing of pregnant women, resulting in missing maternal, fetal, and neonatal outcomes in asymptomatic women.
Conclusion
This study conducted during the first wave of the pandemic demonstrated that most pregnant women in their third trimester who were infected with COVID-19 (Coronavirus) presented mild symptoms and their course of pregnancy and outcome was not affected. The risk of neonatal COVID-19 infection was uncommon. Further studies are needed to confirm maternal-fetal vertical transmission.
Ethical Approval
Ethical approval was taken from the Institutional Review Board (IRB) of Royal Commission Medical Centre, Yanbu, Saudi Arabia.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Nazar T, Aziz B, Shabbir B, Saeed F, Nawaz K, Nabeel M, et al. Predictors of Disease Severity in Adult Covid-19 Patients Admitted in Mayo Hospital, Lahore, Pakistan. Journal of the College of Physicians and Surgeons Pakistan. J Coll Physicians Surg Pak. 2021;31(6):638–643.
- Harrison GA, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Tre Trends Immunol. 2020;41(12):1100–1115.
- Rasmussen SA, Jamieson DJ. Pregnancy, Postpartum Care, and COVID-19 Vaccination in 2021. JAMA. 2021;325(11):1099–1100.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Day M. Covid-19: identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ. 2020;368:m1165.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a Report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239–1242.
- Zambrano LD, Ellington S, Strid P, Galaung RR, Oduyebo T, Tong TV, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morbi Mortal Wkly Rep. 2020;69(44):1641–1647.
- Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann Intern Med .2020;172(11):726–734.
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815.
- Munir SI, Ahsan A, Iqbal S, Aslam S, Tahira T, Alqai S, et al. Fetomaternal Outcome in Women with COVID-19 in a covid Designated Hospital in Lahore, Pakistan. Biomedica. 2020;36(2):228–234.
- Sheikh NA, Khanum F, Erum Pervaiz, Kanwal Nadeem. Maternal and neonatal outcome of vertical transmission in pregnant women during covid-19 pandemic at Quetta, Pakistan. Professional Med J. 2021;28(5):742–748.
- Yu N, Li W, Kang Q, Xiong Z, Wang S, Lin X, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-Centre, descriptive study. Lancet Infect Dis. 2020;20(5):559–564.
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320.
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548.
- Hanna N, Hanna M, Sharma S. Is pregnancy an immunological contributor to severe or controlled COVID-19 disease? Am J Reprod Immunol. 2020;84(5):e13317.
- Trad ATA, Ibirogba ER, Elrefaei A, Narang K, Tonni G, Picone O, et al. Complications and outcomes of SARS-CoV-2 in pregnancy: Where and what is the evidence? Hypertens Pregnancy. 2020;39(3):361–369.
- Ghayda RA, Li H, Lee KH, Lee HW, Hong SH, Kwak M, et al. COVID-19 and Adverse Pregnancy Outcome: A systematic Review of 104 Cases. J Clin Med. 2020;9(11):3441.
- Khalil A, Hill R, Ladhani S, Pattisson K, O’Brien P. Severe acute respiratory syndrome coronavirus 2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol. 2020;223(2):296–297.
- Farooq F, Salahuddin N, Nauman D, Farooq O, Sagheer F, Faruqi NJ, et al. Presentation of pregnant women during COVID -19 pandemic in Farooq Hospital, wood. Journal of Akhtar Saeed Medical & Dental College. 2020;2(2):50–56.
- Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: A preliminary analysis. Am J Roentgenol. 2020;215(1):127–132.
- Yan J, Guo J, Fan C ,Joan J, Yu , Li J, et al. Coronavirus disease 2019 in pregnant women: A report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111.e1–111.e14.
- Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323(18):1848–1849.
- Cosma S, Carosso AR, Cusato J, Borella F, Carosso M, Bovetti M, et al. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am J Obstet Gynecol. 2021;224(4):391.e1–391.e7.
- Huntley BJF, Mulder IA, Di Mascio D, Vintzileos WS, Vintzileos AM, Berghella V, et al. Adverse Pregnancy Outcomes Among Individuals with and Without Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Systematic Review and Meta-analysis. Obstet Gynecol. 2021;137:585–596.
- Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324(7):705–706.
- Zeng L, Xia S, Yuan W, Xiao F, Shao J, Zhou W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722–725.
- Knight M, Bunch K, Vousden N, Morris E, Simpson N, Gale C, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107.
- Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2022;35(8):1619–1622.
Keywords
COVID-19; Coronavirus; Delivery; Pregnancy; Fetal; Neonatal
Cite this article
Malik HS, Khan HP, Ali IB, Al-juhani AK, Seida AR, Sindi H, et al. Effect of COVID-19 infection in pregnancy and perinatal outcome. A case series study. Clin Surg J. 2022;3(2):1–5.
Copyright
© 2022 Humaira Saeed Malik. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



