High Altitude Bilateral Carotid Body Tumor: A Surgical Management
Sudesh Kumar;
Narender Kumar;
Umesh Kumar;
* Niraj Gupta;
Puneet Sharma;
RS Kanwar;
-
Sudesh Kumar: Department of Otolaryngology and Head Neck Surgery, Rajendra Prashad Government Medical College, Himachal Pradesh, India.
-
Narender Kumar: Department of Otolaryngology and Head Neck Surgery, Rajendra Prashad Government Medical College, Himachal Pradesh, India.
-
Umesh Kumar: Department of Otolaryngology and Head Neck Surgery, Rajendra Prashad Government Medical College, Himachal Pradesh, India.
-
* Niraj Gupta: Department of Surgery, Rajendra Prashad Government Medical College, Himachal Pradesh, India.
-
Puneet Sharma: Cardiothoracic and Vascular Surgery, Rajendra Prashad Government Medical College, Himachal Pradesh, India.
-
RS Kanwar: Cardiothoracic and Vascular Surgery, Rajendra Prashad Government Medical College, Himachal Pradesh, India.
-
Apr 07, 2022 |
-
Volume: 3 |
-
Issue: 1 |
-
Views: 1983 |
-
Downloads: 1529 |
Abstract
Carotid body tumor arise from the paraganglionic cells located at the medial surface of the carotid bifurcation. Bilateral tumors occur in only 5% of these cases. Our case report is about a 29-year-old female resident of high altitude (> 3500 meters above sea levels), she developed bilateral neck swelling and her radiological investigations determined she had bilateral carotid body tumors. The larger tumor (Shamblin III) was successfully excised and the smaller tumor was kept under follow-up.
Introduction
Para-Ganglion cells (PG) or neuro-endocrine cells are derived from neural crest and based on location, they are classified as abdominal and extra-abdominal. Tumor arising from these cells are known as Para Ganglomas (PGS). The adrenal gland is the largest abdominal organ containing PG cells, they also contain chromaffin cells, which is pivotal in its functioning as an endocrine organ. The extra-abdominal PGS are located in the Head and Neck (HN) and mediastinum. They contain mostly non-chromaffin cells and may function as chemo-receptors e.g. carotid body tumor.In the HN region, these are found along the parasympathetic nerve, cranial nerve IX and X, and the superior and inferior laryngeal nerves [1]. PGS are benign and highly vascular tumors that arise from paraganglionic cells [2]. About 0.5% of HN tumors are PGS [3]. Around 65% of all head and neck PGS are Carotid Body Tumors (CBT) [4]. In the beginning, it appears as a painless mass in the neck, but it can grow to a considerable size over time, making it life threatening [5].They most often occur unilaterally, while approximately 5% are bilateral in HN region [6]. The radiological appearances of PG are characteristic and are very useful in the diagnosis. Not only do they assist in staging, but they also provide guidance on how to manage them [7]. After a diagnosis is made, surgery is the only definitive treatment, although it has risks and complications [8]. In bilateral tumors, either a staged operation should be performed or a smaller tumor should be observed after resection of the larger tumor [4]. When bilateral resections are done simultaneously, a high rate of cranial nerve injuries and cerebrovascular complications can result, which can have severe consequences [9]. We describe the case of a young female, who was a native of high altitude and had bilateral CBT. The larger tumor of these was surgically removed and she is in follow-up for the tumor of the contra-lateral side.
Case Presentation
A 29-year-old married female patient native of high altitude (11400 feet), in Himalayan state of India, presented to us with complaint of right upper neck swelling for one year duration. It was painless initially but for the last two months there was intermittent pain with mild relief on medication. Around two months ago, she noticed similar swelling on the left side. She denied any family history of similar swelling in the head and neck region. There was no history of palpitation, flushing or fluctuating hypertension. During the physical examination, in the right upper neck, there was a 5 cm x 4 cm non-tender, pulsatile swelling in upper part of anterior triangle of neck, superiorly it was extending under the mandible and inferiorly up to the level of hyoid bone. On the left, there was a 1 cm x 2 cm similar pulsatile swelling at level of hyoid bone. The examination did not revealed any intra-oral bulges and all the cranial nerves were functioning normally. Contrast-Enhancing Computed Tomography (CECT) and CT angiography of the neck revealed a massive mass measuring 6.5 cm x 5 cm x 4.5 cm on the right side (Shamblin III) and 1 cm x 1.5 cm x 1.2 cm on the left side (Shamblin I). The larger right side tumor extended superiorly to the skull base (Figure1, Figure 2).
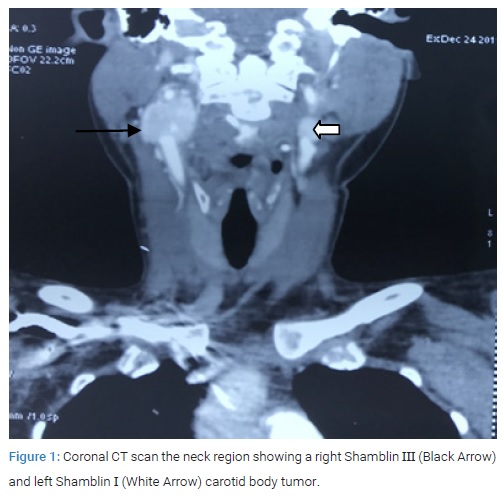
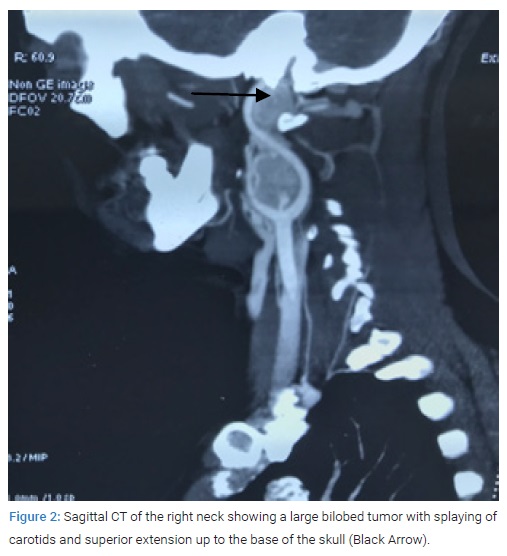
CT chest and abdomen showed no similar lesions. CT head revealed a patent circle of Willis on the contralateral side. This was performed to assess the risk of hemiplegia if during the surgery ICA is temporary clamped for some time. Her vitals were stable and the 24 urinary Vanillyl mandelic acid level was 5 µ/L. After a detailed discussion with the patient and a review of the literature, informed consent for the right CBT excision was obtained. The vascular team was consulted regarding the possibility of carotid artery and subsequent need for repair due to the large size and extension of the tumor to the base of the skull. Nasal intubation was preferred because it aids in exposure by sub-luxating the mandible anteriorly. The incision was made beginning from a point two fingers below the inferior border ramus of the mandible and extended posteriorly to the mastoid process. Sub-platysmal flap was elevated and sternocleido-mastoid muscle was retracted posteriorly and carotid artery sheath was opened. The internal jugular vein was secured and retracted. The vagus, hypoglossal and spinal accessory cranial nerve were identified and preserved. The control of the Common Carotid Artery (CCA), Internal Carotid Artery (ICA), and External Carotid Artery (ECA) was obtained through passing vascular loops. Initial dissection of the tumor from ECA was followed by identification and ligation of the main feeder ascending pharyngeal artery. The stylo-mandibular ligament was cut and the cranial end of the ICA was secured by superior dissection of the tissue around the ICA. In the end, dissection was started on the medial side of carotid bifurcation, where the tumor was found firmly adherent to the vessel wall.
During dissection, Prolene No. 6 was immediately used to patch up two rents accidentally produced in the ICA wall.The tumor was then excised and it was around 6.5 cm x 4 cm x 2 cm in size, bilobed structure, and it was sent for the histopathological examination (Figure 3).
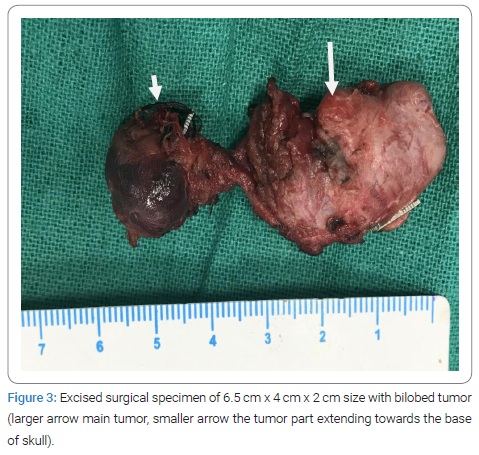
Intra-operative doppler ultrasound showed absence of the pulse in the ICA; and it was suspected to be thrombosed, probably due to manipulation and sutures applied to control bleeding form ICA. As a result, the vascular team opened the ICA, removed the clot that was occluding its lumen, and checked for retrograde bleeding. An end-to-end anastomosis of CCA to ICA was performed due to the risk of re-thrombosis. The anastomosis was performed with continuous sutures of Prolene 6, and vessels were clamped for approximately ten minutes. Patient was immediately heparinised with 3000 units, and application of heparin in this dose continued every 6 hourly for one week. In the intensive care unit, that was brought after surgery, she was kept intubated overnight and extubated after 24 hours. There were no neurological deficits or cranial nerve injuries after surgery. After one week on low dose aspirin, she was discharged and kept on follow-up.
On microscopic examination there were well defined nests if cuboidal cells (Zellballen pattern) separated by highly vascular fibrous septa suggestive of carotid body PG and excised carotid vessel wall did not showed infiltration. CT scan after six month showed right normal patent ICA and stable tumor on left side and she was kept on follow-up.
Discussion
CBT was first described in 1973 by Albrecht Von Haller as ganglion minitum [10]. The three variations are described in the literature as familial, hyperplastic, sporadic (85%), and sporadic usually occurs in the 4th decade of life [11]. Associated with an early presentation and a bilateral CBT rate of 30%–40%, is the familial variant [12]. As seen in our patient, the hyperplastic variant is associated with chronic hypoxia, including chronic pulmonary obstructive disease, cyanotic heart disease, and high altitude (> 5000 feet) [13].
Shamblin in 1971 was first to suggest a grading system for CBT depending upon the size of tumor and encasement of carotid vessels [14]. Grade I refers to small tumor, that show no encasement of carotid vessels, and can be easily dissected. Grade II tumors are adherent or partially encase the carotid vessels and compress them but they can be dissected. Grade III are encasing and adherent requiring vessel resection and reconstruction. Our patient had a Shamblin III tumor on the right side, which is associated with a 30%–46% risk of neurovascular injury [15]. During surgical excision of a CBT tumor, the vagus and hypoglossal nerves are most vulnerable to injury [16]. If both sides of these nerves are injured in bilateral CBT surgery, severe morbidity can result. Consequently, a conservative treatment of smaller tumor either by staging or observing is a better treatment option [4]. Whenever a patient develops postoperative cranial nerve palsy after the removal of larger tumor, it is always recommended to observe the other side tumor by physical examination or radiologically. There is another school of thought, based on their experience, that the smaller tumor should be removed first, followed by the larger ones [9]. The surgical resection of bilateral CBT tumors, however, may result in baroreflex failure post-op, which is characterized by sympatho-excitation, hypertension, and tachycardia [17].
Tumors larger than 6 cm size, the exposure of tumor especially superior or cranial end is always a area of concern. The cranial exposure can be enhanced extension by nasal intubation, anterior sub-luxation of the mandible, and excision of the styloid process and mandibular osteotomy [18]. Similarly, we did naso-tracheal intubation for anaesthesia which was useful for the cranial (superior) exposure as it increases the space between tumor and mandible. It has been reported that 23.2% of ICA injuries occur during CBT surgery and that most of them occur on the medial surface of the carotid bifurcation [19]. The repair of vessel injuries can be performed though direct closure, patch plasty, or graft reconstruction [16]. In our case also the tumor was firmly adherent and accidental tear was managed with lateral suturing but it had lead to ICA thrombosis which was finally managed by end to end anastomatosis of CCA and ICA.
Conclusion
Bilateral Carotid Body Tumor presents a unique diagnostic as well therapeutic challenge. Excision of the larger tumor and observation of the smaller one is well-accepted management option. In tumors of six centimetres or larger, there is a high chances of vascular injury, so a vascular team consultation is utmost important. We also saw that naso-tracheal intubation improves the exposure of tumor by sub-luxating the mandible anteriorly.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Georgiadis GS, Lazarides MK, Tsalkidis A, Argyropoulou P, Giatromanolaki A. Carotid body tumor in a 13-year-old child: Case report and review of the literature. J Vasc Sug. 2008;47(8):874–880.
- Fishbein L. Pheochromocytoma and paraganglioma: genetics, diagnosis, and treatment. Hematol Oncol Clin North Am. 2016;30(1):135–150.
- Lee JH, Barich F, Karnell LH, Robinson RA, Zhen WK, Gantz BJ, et al. American College of surgeons commission on cancer; American cancer society. National cancer data base report on malignant paragangliomas of the head and neck. Cancer. 2002;94(3):730–737.
- Singh A, Subhash A, Panda NK, Mishra AK. Bilateral carotid body tumor in a 13-year-old child: our experience. Journal of head & neckphysician and surgery. 2017;5(2):86–89.
- Sobol SM, Dailey JC. Familial multiple cervical paragangliomas:report of a kindred and review of the literature. Otolaryngol Head Neck Surg. 1990;102(4):382–390.
- Fennessy BG, Kozakewich HPW, Silvera M, Frerichs K, Lillhei CW, Poe D, et al. The presentation and management of multiple paraganglioma in head and neck. Ir J Med Sci. 2011;180(3):757–760.
- Wang SJ, Wang MB, Barauskas TM, Calcaterra TC. Surgical management of carotid body tumors. Otolaryngol Head Neck Surg. 2000;123(3):202–206.
- O Neill S, O Donnell M , Harkin D, Loughrey M , Lee B , Blair P, et al .A 22- year Northern Irish experience of carotid body tumours. Ulster Med J. 2011;80(3):133–140.
- Boedker CC. Paraganglioma and paraganglioma syndrome. GMS Cur Top otorhinolaryngol head and neck surgery. 2011;10:03.
- Kruger AJ, Walker PJ, Foster WJ, Jenkins JS, Boyne NS, Jenkins J. Important observations made managing carotid body tumors during a 25-year experience. J Vasc Surg. 2010;52(6):1518–1523.
- Burgess A, Calderon M, Jafif-Cojab M, Jorge D, Balanza R. Bilateral carotid body tumor resection in a female patient. Int J surgery case reports. 2017;41:387–391.
- Demir T, Uyar I, Demir HB, Sahin M, Gundogdu G. Five-year follow-up of a patient with bilateral carotid body tumors after unilateral surgical resection. Am J Case Rep. 2014;15:426–430.
- Sajid MS, Hamilton G, Baker DM. A multicenter review of carotid body tumour management. Eur J Vas Endovas Surg. 2007;34(2):127–130.
- Shamblin WR, ReMine WH, Sheps SG, Harrison EG Jr. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122(6):732–739.
- Lim JY, Kim J, Kim SH, Lee S, Lim YC, Kim JW, et al. Surgical treatment of carotid body paragangliomas: outcomes and complications according to the shamblin classification. Clin Exp Otorhinolaryngol. 2010;3(2):91–95.
- Sen I, Stephen E, Malepathi K, Agarwal S, Shyamkumar NK, Mammen S. Neurological complications in carotid body tumors: a 6-year single-center experience. J Vasc Surg. 2013;57(2 Suppl):64S–68S.
- Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, et al. Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension. 2015;65(6):1365–1371.
- Prouse G, Mazzaccaro D, Settembrini F, Carmo M, Biglioli F, Settembrini PG. Double osteotomy of mandibula in the treatment of carotid body tumors with skull base extension. J Vasc Surg. 2013;58(2):486–490.
- Anand VK, Alemar GO, Sanders TS. Management of the internal carotid artery during carotid body tumor surgery. Laryngoscope. 1995;105(3 Pt 1):231–235.
Keywords
Carotid body tumor; Bilateral para-ganglioma; High altitude; Resection
Cite this article
Sudesh K, Narender K, Umesh K, Gupta N, Sharma P, Kanwar RS. High altitude bilateral carotid body tumor: A surgical management. Clin Surg J. 2022;3(1):1–4.
Copyright
© 2022 Niraj Gupta. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).



