Outcomes of Transanal Endoscopic Surgery for Rectal Polyps
* Mathiasen M;
Jaensch C;
Worsoe J;
Madsen AH;
-
* Mathiasen M: Department of Surgery, Regional Hospital Herning, Denmark.
-
Jaensch C: Department of Surgery, Regional Hospital Herning, Denmark.
-
Worsoe J: Department of Surgery, Regional Hospital Herning, Denmark.
-
Madsen AH: Department of Surgery, Regional Hospital Herning, Denmark.
-
Feb 18, 2022 |
-
Volume: 3 |
-
Issue: 1 |
-
Views: 3589 |
-
Downloads: 1741 |
Abstract
Background & Aims: Transanal Endoscopic Microsurgery (TEM) is a procedure for en-bloc removal of polyps in the rectum. Reported recurrence and complication rates are varying and optimal postoperative surveillance program is still not well-defined. The aim of this study was to evaluate recurrence and complications after polyp removal, in order to evaluate the quality of TEM.
Material and Methods: A single center experience conducted at Regional Hospital Herning, Denmark. Study population was patients undergoing ICD-10 code KJGA75: “Rectoscopic microsurgical excision of pathological tissue in the rectum” from June 01, 2016 to May 31, 2019. Patients were identified using The Danish Civil Registration System (CPR). Data were obtained through patient files. Outcomes were recurrence stated as histopathological recurrence at early check-up five months after TEM and complications defined as early and late, lasting less or more than 30 days postoperatively, respectively.
Results: One hundred twelve patients underwent TEM. Ninety-seven attended early check-up after a mean of 128 days (95% CI: 119.4–136.8). Seven patients (7.2%) were diagnosed with recurrence, none with cancer. Six out of seven recurrences had free resection margins. One had TEM resection again and seven were treated endoscopically. 30 (27%) patients experienced complications. Twenty-two early complications, primary bowel incontinence, urinary retention and postoperative bleeding and eight late complications, mainly bowel incontinence to feces or flatus.
Conclusion: Recurrence and complications occurred in 7% and 27% of the patients, respectively. A major finding was, that six out of seven recurrences had free resection margins underlining the importance of early check-up regardless of resection margin status. No interval cancers recurred during the normal control program indicating, that five months is a sufficient follow-up interval for a first check-up.
Abbreviations
TEM: Transanal Endoscopic Microsurgery; TRUS: Trans Rectal Ultra Sound, MRI: Magnetic Resonance Imaging; CT: Computed Tomography; CPR: The Danish Civil Registration System; ESD: Endoscopic Submucosal Dissection; FTR: Full Thickness Resection; BMI: Body Mass Index.
Introduction
It is well-known that rectal neoplasms are premalignant, and 2.5% transform to malignancy per year [1,2]. Therefore, all polyps should be removed followed by a surveillance program to detect recurrence. TEM is a minimally invasive technique which allows precise resection of rectal tumors located four to 18 cm from the anal verge [3]. The technique combines endoscopy and laparoscopy, is tissue-sparing and protects the anal sphincter [4]. It is known, that TEM is associated with less perioperative mortality and morbidity compared to transabdominal surgical approaches regarding anorectal, bladder and sexual function [5]. Indications for TEM are removal of adenomas and certain low risk T1 carcinomas [4–6]. Additionally, TEM might be chosen as treatment of T2 rectal cancer in patients with severe comorbidity were laparoscopy or laparotomy is contraindicated. Furthermore, TEM as part of palliative treatment is an option [7–10]. Rigid proctosigmoidoscopy is performed to assess position and accessibility of the polyp. TRUS (TransRectal Ultrasound Examination) has been shown to be more than 90% sensitive in determining the depth of penetration of rectal cancer [11]. In order to stage a polyp preoperatively, TRUS and MRI can be used to determine depth of penetration and lymph node status. Optimal surveillance program after TEM is still not well-defined and risk of adenoma recurrence is uncertain [12]. The aim of this study was to asses adenoma recurrence rate at 5 months check-up after TEM in order to evaluate, if this interval is sufficient for surveillance. Secondary, complications related to the procedure were assessed.
Material and Methods
This study was a single center experience, conducted at Department of Surgery, Herning, Denmark. Study population was defined as patients undergoing TEM with ICD-10 code KJGA75: “Rectoscopic microsurgical excision of pathological tissue in rectum”, during three years from June 1, 2016 to May 31, 2019. Patients were identified using CPR. Patient files and histopathological examinations were retrieved from the electronic patient file system. Data with regard to the following patient features were retrieved: Sex, age, BMI and indication for TEM. TEM data regarding date of surgery, position of the patient during surgery, duration, resection type (TEM assisted Endoscopic Submucosal Dissection (ESD) or TEM assisted Full Thickness Resection (FTR), duration of hospitalization. Macroscopic and histopathologic polyp characteristics including distance from the anal verge, size, macroscopic appearance, Paris classification, histopathologic free resection margins, type of adenoma (serrated, tubular, tubulovillous, or villous) or adenocarcinoma. Complications were divided into two categories: Early postoperative complications within 30 days (bleeding, abscess, urinary retention, and perforation) and late complications developing after or lasting longer than 30 days after TEM, for example incontinence to feces or flatus and stenosis. Recurrence was defined as histopathological recurrence of adenoma or adenocarcinoma at previous resection site at early check-up, usually with-in 5 months after TEM. Patients referred to TEM underwent preoperative digital examination, sigmoidoscopy, rectoscopy, TRUS, and colonoscopy if not already performed. Indications for TEM at our department were: Polyp size of at least two cm, residual or recurrence of adenomatous tissue following endoscopic polypectomy in the rectum (defined as 0 cm to 15 cm from the anal verge), or removal of polyp site after endoscopic removal of a malignant polyp.
Patients were excluded from the study if TEM was converted to an abdominal surgical approach. At day of TEM patients had rectal enema. Antibiotic prophylaxis for gram-negative and anaerobic strains (gentamicin and metronidazole) was administered at the beginning of anesthesia. The procedures were performed using TEM (Richard Wolf®, Knittlingen, Germany) under general anesthesia. TEM was performed either as TEM assisted ESD or FTR. FTR was chosen where submucosal dissection was not possible (re-resection in scar tissue) or not advisable (resection of polyp site after endoscopic removal of a malignant polyp). Further details about surgical technique of TEM are described elsewhere [1,8]. All cases except one were confirmed macroscopically free from polyps by the surgeon at the end of TEM procedure. There were no postoperative restrictions, and patients were discharged when there was no suspicion of complications or fever. Regular controls were planned as sigmoidoscopies 5 months after TEM. Intensified control programs due to adenocarcinomas were individualized and included different combinations of procto, sigmoido and colonoscopies, MRI and CT-scans. The study was defined as a quality management project which was approved by the hospital management and no further ethical approvements were needed. Procedures were followed in accordance with the Helsinki Declaration of 1975.
Data were administered electronically in REDCap (Vanderbilt University, Nashville, TN, USA) [13,14]. Data analysis was performed using Stata (version 14.2; StataCorp LP, Texas, USA). Normality assumptions were confirmed using histograms and QQ‐plots. Normally distributed data were described as means with 95% confidence intervals, data which were not normally distributed were stated as median and range. Missing data were excluded from analysis.
Results
One hundred twelve patients, 70 men and 42 women underwent TEM during the study period. Mean age was 68 years (95% CI: 66.6–70.0) and median BMI 26.9 (range 18.5–49.4). TEM related data are shown in (Table 1). None of the patients suffered from hereditary polyposis syndromes. Polyp placement was equally distributed between anterior, posterior, left and right with 27,31,22 and 26 patients respectively. Regardless of that, 40% underwent TEM in lithotomy position, 22% in prone jackknife and 21% and 15% in right and left lateral decubitus respectively, after individual assessment of the surgeon. Paris classification was stated in 37 patients. Infiltration of the polyp was assessed by TRUS. Histopathological characteristics of the polyps can be found in (Table 2).
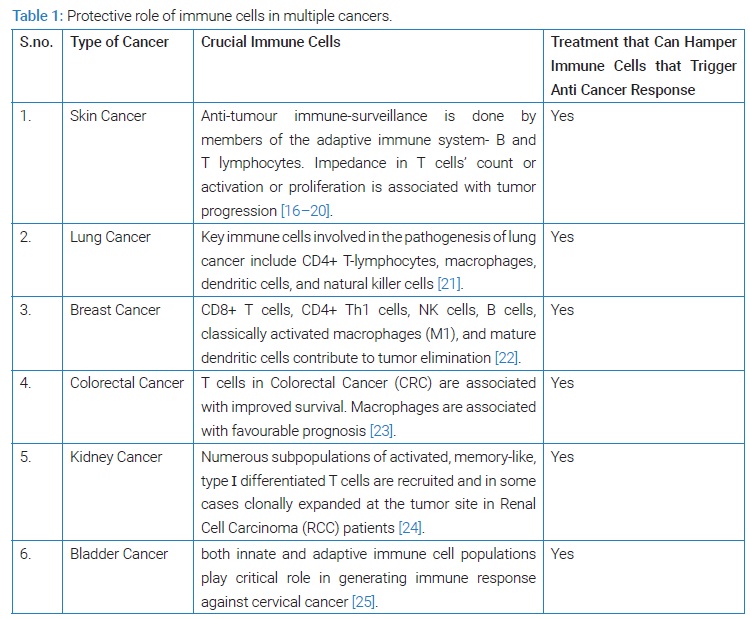
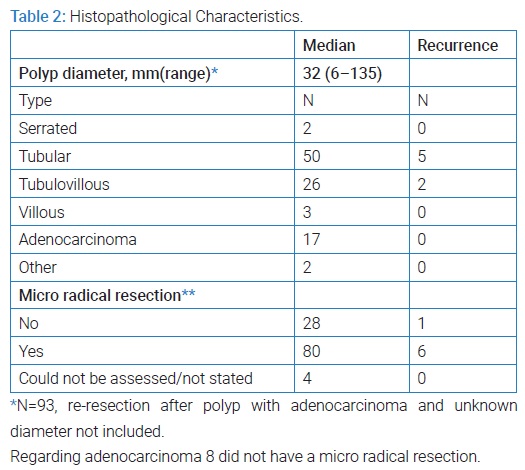
Control Program and Recurrence: The following results include all indications for TEM. Ninety-seven (86.6%) attended early check-up as part of a scheduled control program after a mean of 128 days (95% CI: 119.4–136.8) after TEM.
After first check-up 81 (72.3%) underwent regular control programs (consisting of regular colonoscopies according to the departments guideline). 6 (5.4%) underwent other control programs because of other cancer, dilatations because of stenosis or more polyps to be removed with TEM. Twenty-two patients had cancer of whom 10 (8.9%) underwent intensified control programs and 12 (10.7%) went directly to cancer treatment according to DCCG’s (Danish Colorectal Cancer Group) guideline [15]. Three patients did not attend control; one died of other causes before scheduled control, one dropped out of the program due to operation for prostate cancer and one patient did not wish to participate after several failed attempts of rectal enemas. The 97 patients undergoing early check-up as part of the scheduled control program seven patients (7.2%) experienced recurrence at follow-up. Recurrence was on average diagnosed after 185 days (105 days–239 days). Recurrence occurred among five tubular and two tubulovillous adenomas. One patient had repeat TEM resection, the six remaining were treated endoscopically. Interestingly, five of the patients with recurrence of adenomatous tissue were free from adenomas after the second procedure. Only two patients needed additional procedures for a second recurrence. All patients are still undergoing control programs.
Complications: A total of 30 (26.8%) patients experienced complications. In total 28 were classified as Clavien-Dindo I-II, and two Clavien-Dindo III. Twenty-two were categorized as early complications and eight as late. Regarding early complications; Seven (6.3%) had urinary retention, six only lasted 24 hours and one lasted one week. Three (2.6%) had bowel incontinence to either gases or feces ceasing within two weeks. Rectal bleeding more than expected, was registered among three (2.6%). All cases were self-limiting within few days not requiring blood transfusion or surgery. Two (1.8%) perforations were registered. One was treated conservatively with antibiotics and discharged the day after TEM. The other was detected 3 days postoperatively, the perforation was treated laparoscopically with direct suture and diverting stoma, which was reverted 6 months later. Seven early complications were categorized as others; four had fevers without focus, one cystitis, one had more pain than expected and one possible micro perforation treated with antibiotics. Considering the eight late complications, six (5.4%) had bowel incontinence to either gases or feces. All of them were registered among patients who underwent FTR. Four ceased within one year, in the two remaining cases data on incontinence are missing after 5 months. Additionally, there was one patient with urinary retention who was referred to urological examination and introduced to catheterization. One developed stenosis after resection of a circumferential adenoma, requiring dilatation several times.
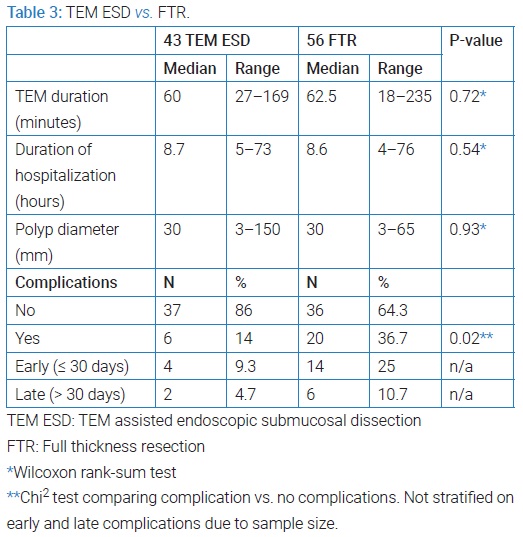
TEM assisted ESD vs. FTR: In patients with primary polyp or residual/recurrence of polyp after endoscopic polypectomy, TEM surgery was performed either as TEM assisted endoscopic submucosal dissection (TEM ESD) or (FTR). We found, that FTR was associated with significantly more complications than TEM ESD. Details on procedure duration, hospitalization, polyp size and complications can be found in (Table 3), in which the indication re-resection after polyp with adenocarcinoma is not included as 10 of these underwent FTR and only one ESD.
Cancer: Out of 112,12 patients underwent TEM on indication removal of polyp site after endoscopic removal of a malignant polyp and two on other indication. Ninety eight patients underwent TEM without any prior knowledge of malignancy equal to the indication removal of primary polyp or a residual/recurrence polyp after polypectomy. Among these, 17 cases of cancer were found. For pathological information see (Supplementary Table 1).
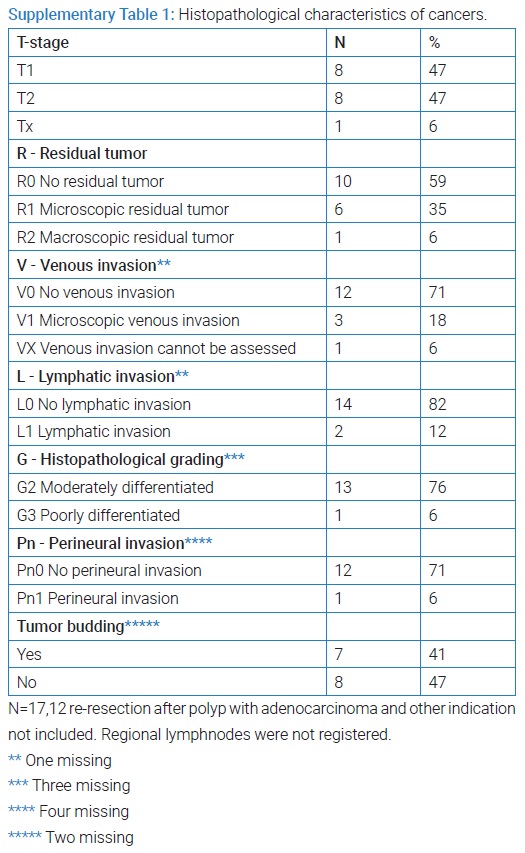
Ten of these were not removed completely (resection margin < 1mm) and underwent consecutive completion resection. The other seven entered into an intensified control program. One of these had progression on MRI one year after TEM and underwent total mesorectal excision. Microscopy assessment showed pT3N1V1 tumor. Additionally, 12 patients were treated with TEM because of unexpected cancer in a polyp resected endoscopically. None of these had residual cancer in the TEM specimen. Further data from the underlying research material can be obtained upon request to the corresponding author.
Discussion
One-hundred and twelve patients underwent TEM. 7.2% had recurrence of the adenomatous tissue after a median of 185 days (105 days–239 days), and none of the recurrences were malignant. Thirty patients (26.8%) had complications, 22 were early complications and eight were late complications. Two bowel perforations were detected of which one needed surgery. FTR was related to greater variation in and significantly more complications than TEM assisted ESD. Regarding duration of TEM and hospitalization, we found no statistically significant difference between resection types. No significant difference in polyp size was found between resection types. A greater variance in polyp size among ESD could be related to improvement in TEM technique over time, as ESD has become more utilized, and the surgeons are able to resect larger polyps over time.
Seventeen cases of cancer were detected of which ten underwent immediate cancer surgery and seven were followed closely (interval). A strength of this study consisted in the population size and short study period diminishing changes due to improvement of TEM technique during time. Moreover, data were obtained via CPR. Early check-ups were all performed at our department, hence patient files were all available. Data from patients referred from other hospitals were all available because of a shared electronic patient file system within the Central Region of Denmark. Almost all patients were operated by only two surgeons, ensuring that the patients underwent a uniform treatment by experienced surgeons. This might reduce inter-surgeon variability. On the other hand, this study includes data from a period of three years making the surgeons more experienced during the study period possibly diminishing complication rate over time. A tendency of increased use of TEM assisted ESD rather than FTR during the study period will probably further diminish complications during the study period. This study is limited by a short follow-up period; hence we are only capable of evaluating TEM on short term. On the contrary, almost all patients underwent 5th month check-up diminishing selection bias.
The recurrence rate of 7.2% in this study is low compared to the medical literature. Two studies found a recurrence rate of 13.8% and 15% with a follow-up period of 623 days and 44 months respectively [2,5]. Two reviews from 2014 and 2020 respectively found recurrence rate of TEM varying from two to 16% and 0% to 22.7% [16,17]. Two other studies found lower recurrence rates, one study only had one out of 158 patients and another with recurrence among 4%, in which follow-up time is not clearly defined [10,18]. Our recurrence rate could possibly be due to a relatively short follow-up period. McCloud et al. and Whitehouse et al. found that incomplete resection margins had a higher risk of recurrence [12,19]. This is not the case in our study, since risk of recurrence for the patient group considered in this study was 1/19 (5.3%) and 6/74 (8.1%) for incomplete and complete resections margins, respectively. Recurrence could possibly be due to seeding, which could justify early check-up. Most recurrences can be treated endoscopically or with TEM [2]. This is comparable with our study as only one of seven recurrences needed repeat TEM. Regarding complications, urinary retention (7.1%) and incontinence to feces and/or flatus (5.4%) were the most frequent complications in our study, and most were detected among FTR. It is known that FTR is more frequently related to postoperative pain, sepsis and perforation, which was also found in this study [20]. Two cases with urinary retention needed urologist referral. Laliberte et al. found postoperative urinary retention among 19% of which most were managed with temporary single catheterizations or Foley catheter, indicating that this is a common and transitory complication [21]. Tsai et al. reported urinary retention among 10.8%, but they did not include single catheterizations [22]. This result is comparable with our study. A total of nine patients (8%) had bowel incontinence to feces or gasses, seven ceased within one year, on the remaining two, data on this complication is missing after one year, hence it is unknown if the complication is still ongoing. Fecal incontinence was detected among 4.1% by Tsai et al. but incontinence to gases was not included. Bowel incontinence is invalidating both regarding gasses and feces, and possibly two patients still suffered from that after one year. This is an important complication to have in mind, when TEM is done on a benign indication. Postoperative bleeding occurred in 2.6% in our study and stopped spontaneously within few days. Thus, incidence and severity of bleeding in our study is much lower than the medical literature, who reports incidence of bleeding up to 6.3% [2,5,19,21].
Conclusion
Transanal Endoscopic Microsurgery (TEM) is a secure method for polyp removal in the rectum regarding both recurrence rate and complications. The recurrence rate of benign polyps after TEM was 7.2% at five months follow-up, and this is comparable with the medical literature. A very interesting finding was, that six out of seven recurrences had free resection margins after initial TEM. This confirms the necessity for early check-up regardless of resection margin status. Furthermore, no recurrence cases included cancer, which assures both a sufficient quality of TEM and follow-up time for first check-up. Complication rates in this study are comparable with those reported in other studies. Most complications were mild and temporary even though bowel incontinence is a non-neglectable complication on the long term. Regarding severe complications, there were two perforations, and only one needed surgery. Urinary retention and fecal incontinence were associated with FTR rather than TEM assisted ESD. No postoperative bleeding needed intervention. Incontinence to feces is high in our study, but it is caused by inclusion of incontinence to flatus as well.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Winawer SJ. Natural History of Colorectal Cancer. Am J Med. 1999;106(1A):3S-6S.
- Chan T, Karimuddin AA, Raval MJ, Phang PT, Tang V, Brown CJ, et al. Predictors of rectal adenoma recurrence following transanal endoscopic surgery: a retrospective cohort study. Surg Endosc. 2020;34(8):3398–3407.
- Demartines N, Von Flue MO, Harder FH. Transanal endoscopic microsurgical excision of rectal tumors: indications and results. World J Surg. 2001;25(7):870–875.
- Amann M, Modabber A, Burghardt J, Stratz C, Falch C, Buess GF, et al. Transanal endoscopic microsurgery in treatment of rectal adenomas and T1 low-risk carcinomas. World J Surg Oncol. 2012;10:255.
- Ganai S, Kanumuri P, Rao RS, Alexander AI. Local recurrence after transanal endoscopic microsurgery for rectal polyps and early cancers. Ann Surg Oncol. 2006;13(4):547–556.
- Allaix ME, Arezzo A, Morino M. Transanal endoscopic microsurgery for rectal cancer: T1 and beyond? An evidence-based review. Surg Endosc. 2016; 30(11):4841–4852.
- Saclarides TJ. Transanal Endoscopic Microsurgery. Clin Colon Rectal Surg. 2015;28(3):165–175.
- Issa N, Murninkas A, Schmilovitz-Weiss H, Agbarya A, Powsner E. Transanal Endoscopic Microsurgery After Neoadjuvant Chemoradiotherapy for Rectal Cancer. J Laparoendosc Adv Surg Tech A. 2015;25(8):617–624.
- Turler A, Schafer H, Pichlmaier H. Role of transanal endoscopic microsurgery in the palliative treatment of rectal cancer. Scand J Gastroenterol. 1997;32(1):58–61.
- Buess G, Mentges B, Manncke K, Starlinger M, Becker HD. Technique and results of transanal endoscopic microsurgery in early rectal cancer. Am J Surg. 1992;163(1):63–69.
- Bipat S, Glas A, Slors F, Zwinderman A, Bossuyt P, Stoker J, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232(3):773–783.
- McCloud JM, Waymont N, Pahwa N, Varghese P, Richards C, Jameson JS, et al. Factors predicting early recurrence after transanal endoscopic microsurgery excision for rectal adenoma. Colorectal Dis. 2006;8(7):581–585.
- Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The redcap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. et al. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- DCCG. dk’s nationale retningslinjer for diagnostik og behandling af kolorektalcancer. 2001.
- Naughton AP, Ryan EJ, Bardon CT, Boland MR, Aherne TM, Kelly ME, et al. Endoscopic management versus transanal surgery for early primary or early locally recurrent rectal neoplasms-a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35(12):2347–2359.
- Heidary B, Phang TP, Raval MJ, Brown CJ. Transanal endoscopic microsurgery: a review. Can J Surg. 2014; 57(2):127–138.
- Issa N, Fenig Y, Schmilowitz-Weiss H, Khatib M, Bachar G, Gingold-Belfer R, et al. Simultaneous local excision of synchronous rectal polyps by transanal endoscopic microsurgery. Eur J Gastroenterol Hepatol. 2020:32(1):45–47.
- Whitehouse PA, Tilney HS, Armitage JN, Simson JNL. Transanal endoscopic microsurgery: risk factors for local recurrence of benign rectal adenomas. Colorectal Dis. 2006;8(9):795–799.
- Yap K, Mills S, Thomas M, Moore J. Submucosal dissection has advantages over full-thickness transanal endoscopic microsurgery in selected rectal lesions. ANZ J Surg. 2017;87:903–907.
- Laliberte AS, Lebrun A, Drolet S, Bouchard P, Bouchard A. Transanal endoscopic microsurgery as an outpatient procedure is feasible and safe. Surg Endosc. 2015;29(12): 3454–3459.
- Tsai BM, Finne CO, Nordenstam JF, Christoforidis D, Madoff RD, Mellgren A, et al. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum. 2010;53(1):16–23.
Keywords
Transanal endoscopic microsurgery; Endoscopic mucosal resection; Polyps; Adenoma; Adenocarcinoma; Recurrence
Cite this article
Mathiasen M, Jaensch C, Worsoe J, Madsen AH. Outcomes of transanal endoscopic surgery for rectal polyps. Clin Surg J. 2022;3(1):1–6.
Copyright
© 2022 Mathiasen M. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).




