Enhanced Recovery after Surgery (ERAS®) for Gastrointestinal Surgery in Africa: A Systematic Review and Meta-Analysis
* George Kefa Kanani;
Jasmine Aboubakar Mrisho;
Xu Zhaohui;
Chen Xin;
-
* George Kefa Kanani: Department of Hernia and Colorectal Surgery, The second Affiliated Hospital of Dalian Medical University, Liaoning Province, China.
-
Jasmine Aboubakar Mrisho: Department of Emergency, Bugando Consultant and Referral Hospital, Mwanza, Tanzania.
-
Xu Zhaohui: Department of Hernia and Colorectal Surgery, The second Affiliated Hospital of Dalian Medical University, Liaoning Province, China.
-
Chen Xin: Department of Hernia and Colorectal Surgery, The second Affiliated Hospital of Dalian Medical University, Liaoning Province, China.
-
Dec 07, 2021 |
-
Volume: 2 |
-
Issue: 3 |
-
Views: 3782 |
-
Downloads: 1912 |
Abstract
Background: Although Enhanced Recovery after Surgery (ERAS®) programs has been proven beneficial in many fields of surgery yet they are not widely practiced in developing countries as compared to developed countries.
Objectives: To evaluate the feasibility and safety of Enhanced Recovery after Surgery (ERAS®) Programs in patients undergoing gastrointestinal surgeries in Africa.
Methods: We conducted a comprehensive systematic search from the following electronic databases: PubMed, the Cochrane library, ClinicalTrials.gov, African Journals Online (AJOL), and African Index Medicus through March 28, 2020. The primary outcome measures were the length of hospital stay, postoperative complications and re-admission rate. Combined overall effect sizes were calculated using fixed-effects or random-effects models.
Results: We identified eight comparative studies reporting a total of 451 patients evaluating outcomes of Enhanced Recovery after Surgery (ERAS®) (n = 224) and Non-ERAS® (n = 227) in patients undergoing gastrointestinal surgeries. The results of the meta-analysis showed that the ERAS® group had shorter hospital stay (MD: -4.04, 95% CI: -4.94 to -3.14,) and fewer Postoperative complications (RR: 0.56, 95% CI: 0.41 to 0.77,) compared with the Non-ERAS® group. However, there was no significant difference between ERAS® group and Non-ERAS® group with regards to re-admission rates (RR: 1.10, 95% CI: 0.49 to 2.48, P = 0.82).
Conclusions: Our meta-analysis (level 2 evidence) showed that the Enhanced Recovery after Surgery (ERAS®) Practices in African settings has similar advantages of shorter hospital stay and less postoperative complications as in developed countries. Progressive adoption of ERAS® in routine surgical practices would benefit many patients undergoing gastrointestinal surgery procedures in African countries.
Introduction
Enhanced Recovery after Surgery (ERAS®) is an evidence-based, multimodal and multidisciplinary perspective to the care of the surgical patient. This perspective was first conceptualized by a Danish surgeon, Henrik Kehlet, in the early1990s [1]. Enhanced Recovery after Surgery (ERAS®) protocols are multimodal perioperative care pathways designed to achieve early recovery after surgical procedures by maintaining pre-operative organ function and reducing the profound stress response following surgery. The key elements of ERAS® protocols include preoperative counseling, optimization of nutrition, standardized analgesic and anesthetic regimens and early mobilization [2,3]. The combination of these clinical pathways have resulted to a significant reduction in length of hospital stay, postoperative complications, re-admission rates, morbidity and mortality, and hospitalization cost, at the same improving postoperative recovery [4,5]. Since its introduction, there is growing evidence that ERAS® programs have been proven beneficial to many surgical specialties including colorectal, hepatobiliary, gastric, pancreatic, as well as to non-gastrointestinal disciplines such as orthopedics, cardiothoracic surgery, obstetrics, gynecology, and anesthesia [6–9]. Implementation of ERAS® programs across variety of surgical specialties has resulted to improved patient outcomes including early mobilization, few postoperative complication rates and shorter length of stay. Although Enhanced Recovery after Surgery (ERAS®) programs have been proven beneficial in many fields of surgery yet they are not widely practiced in developing countries as compared to developed countries [10]. There is restricted number of studies recording successful application of ERAS® in Africa. An updated and comprehensive assessment of the evidence concerning practice of Enhanced Recovery after Surgery in African countries is lacking. The aim of our study was to perform a comprehensive systematic review and possible meta-analysis to evaluate the feasibility and safety of Enhanced Recovery after Surgery (ERAS®) Programs in patients undergoing gastrointestinal surgeries in Africa.
Methods
Design
The study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11].
Inclusion and Exclusion Criteria
Inclusion criteria:
- RCTs and comparative observational studies comparing Enhanced Recovery after Surgery (ERAS®) and Non-ERAS® in patients undergoing gastrointestinal surgery procedures;
- Studies illustrating the ERAS® program with a clear protocol;
- Studies with at least one of the following outcomes; length of hospital stay, rates of postoperative complications, and readmissions.
The exclusion criteria were:
- Studies which are reviews, letters, cases, or bioinformatics;
- Studies including single cohort or not employing control group;
- Studies only have the abstracts presented at national or international conferences without full-text articles;
- Studies describing ERAS® program lacking a clear protocol;
Two independent authors used predefined criteria to identify all included studies. Difference arising between two authors was solved by discussion through the help of another independent third author.
Types of interventions
The intervention was Enhanced Recovery after Surgery (ERAS®) program which was characterized by principles of preoperative counseling, optimization of nutrition, standardized analgesic and anesthetic regimens and early mobilization in gastrointestinal surgical procedures. The primary intervention was compared with conventional gastrointestinal surgical procedures not employing ERAS® protocol.
Outcomes
Primary outcomes were defined as the duration of hospital stay, postoperative complications and re-admission rate.
Literature search strategy
Two authors independently searched the following electronic databases: PubMed, the Cochrane library, ClinicalTrials.gov, African Journals Online (AJOL), and African Index Medicus. The literature search was conducted based on Keywords and Medical Subject Heading (MeSH) terms related to Enhanced Recovery after Surgery program which included Enhanced Recovery after Surgery, “multimodal perioperative care,” “fast track surgery,” “multimodal analgesia,” “Enhanced Recovery after Surgery Protocol,” “ERAS®,” and “FTS” and included gastrointestinal procedures. The name of the African country in the language relevant to this country was also applied. The search was limited to studies in English language. Last search was March 28, 2020. Full search strategy in additional file 1 for PubMed that was adapted to fit with other electronic databases. Furthermore, the reference lists of relevant articles were explored for potential of eligible studies. Finally, a hand search to the relevant African journals in general surgery. Any disagreement arising during search process was settled through the help of a third independent author.
Selection of studies
The title and abstract of identified articles were evaluated by two independent authors. Subsequently, if relevant, the full texts of identified articles were retrieved and evaluated against the eligibility criteria of our study. Those studies that met our eligibility criteria were included. Discrepancies in this process were resolved by discussion between the authors. However, if the disagreement still existed, an independent author was consulted.
Data extraction and management
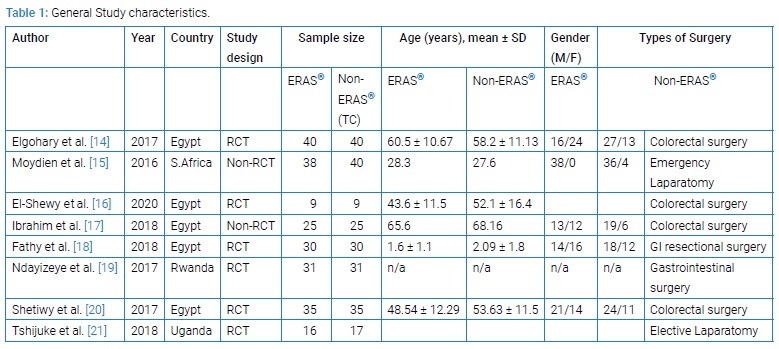
An electronic data extraction spreadsheet was created for intervention reviews. This spreadsheet was pilot-tested in randomly selected articles and adjusted accordingly. The following data was extracted from the included studies: first author, publication year, country of origin, study design, study size, age, Gender and type of surgical procedure and primary outcome data. Any disagreement arising during data extraction process was settled through the help of a third independent author.
Assessment of risk of bias
The quality and risk of bias assessment were carried out by two authors using the Cochrane’s tool [12] and the Newcastle-Ottawa Scale (NOS) [13] for RCTs and observational studies, respectively. The Cochrane’s tool classifies studies into low, unclear and high risk of bias following evaluating and determining the risk of selection bias, performance bias, detection bias, attrition bias, reporting bias and other sources of bias. The NOS is a star-based scoring system (maximum score 9) which enables review authors to evaluate an observational study in the following aspects: the selection of the study groups, the comparability of the groups and the ascertainment of outcome of interest. Studies with score of nine stars were deemed to be at low risk of bias, studies with score of seven or eight stars were deemed to be at medium risk of bias, and those that scored six or less were judged to be at high risk of bias. Any disagreement arising during risk assessment process was settled through the help of a third independent author.
Data synthesis and data analysis
We performed data synthesis as well as analysis using Review Manager 5.3 software. One independent review author entered the extracted data into Review Manager 5.3 software for data synthesis. The entered data were subsequently checked by a second review author. The random-effects model or the fixed-effects models were used, as appropriate, for analysis. I2 tests and Chi-squared test were utilized to determine the heterogeneity of clinical trial results to further decide the model for analyses. When the I2 test value was > 50% and Chi-squared test P-value was <.05, heterogeneity was defined to be high and the random-effects model was utilized. When the I2 test value was less than 50% and Chi-squared test P-value was larger than.05, heterogeneity data were defined to be acceptable and the fixed-effects model was utilized. Mean ± standard deviation and Mean Difference (MD) were used to express and analyze continuous variables, respectively. Categorical data were presented as percentages and analyzed by Relative Risk (RR) or Odds Ratio (OR). Postoperative complication and readmission rate were analyzed by RR and 95% CI while hospital stay was analyzed by MD and 95% CI. Moreover, where more than ten studies were available in analysis of an outcome parameter, funnel plots were planned to be constructed in order to assess their symmetry to visually evaluate publication bias. We conducted sensitivity analyses to explore potential sources of heterogeneity and assess the robustness of our results.
Results
Study characteristics
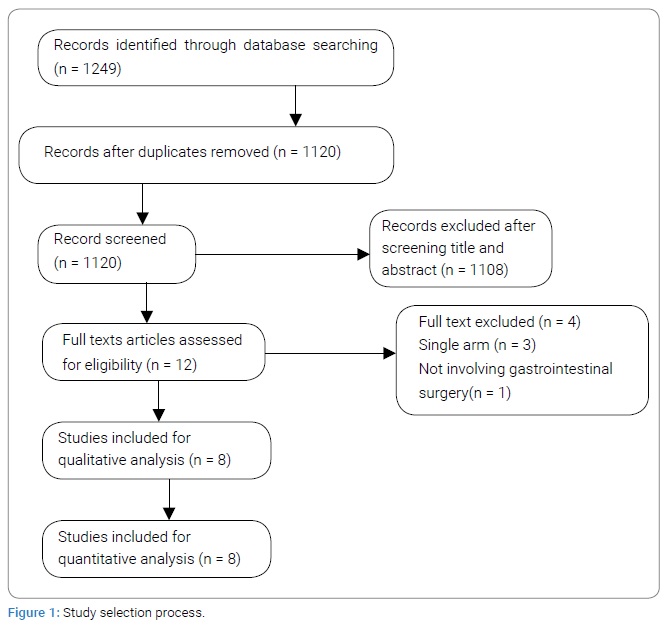
A total of eight studies [14–21] met the inclusion criteria. (Figure 1) shows a flowchart of studies from the initial results of the publication searches to final inclusion or exclusion according to the PRISMA statement. Six articles were RCT and two were non-RCT clinical studies. Overall, 451 patients were included in the analysis. Of these, 224 (49.7%) underwent ERAS®, 227 (50.3%) Non-ERAS®, (Table 1). Table 1 summarizes the main characteristics of the included studies. The countries of studies included Egypt, Uganda, South Africa and Rwanda. The mean age of all the included studies was more than 50-year-old (Figure 1).
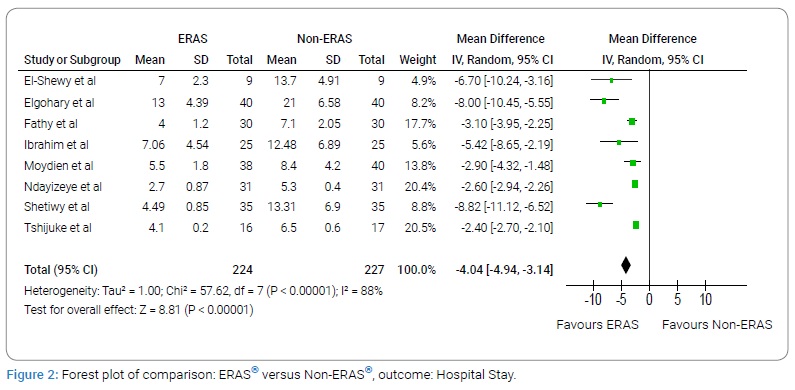
Hospital stay
All eight studies with 224 subjects in the ERAS® group and 227 subjects in the Non-ERAS® group provided data on Hospital stay. Based on the I2 test (I2–88%) and Chi-squared test (P = .000), we chose the random effects model to analyze hospital stay due to high variability. The pooled results showed that the ERAS® group had shorter hospital stay compared with the Non-ERAS® group (MD: -4.04, 95% CI: -4.94 to -3.14, P < 0.00001, (Figure 2)).
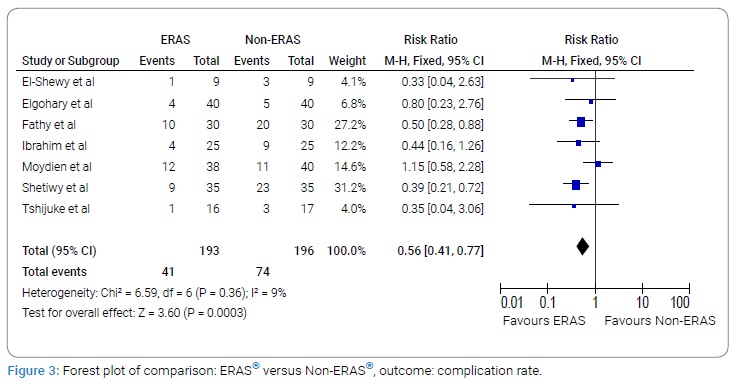
Complication rate
A total of seven studies with 193 subjects in the ERAS® group and 196 subjects in the Non-ERAS® group provided data on Complication rate. Based on the I2 test (I2–9%) and Chi-squared test (P = 0.36), we chose the fixed effects model to analyze Complication rate due to low variability. The pooled results showed that the ERAS® group had fewer Complication rate compared with the Non-ERAS® group (RR: 0.56, 95% CI:0.41 to 0.77, P = 0.0003, (Figure 3)).
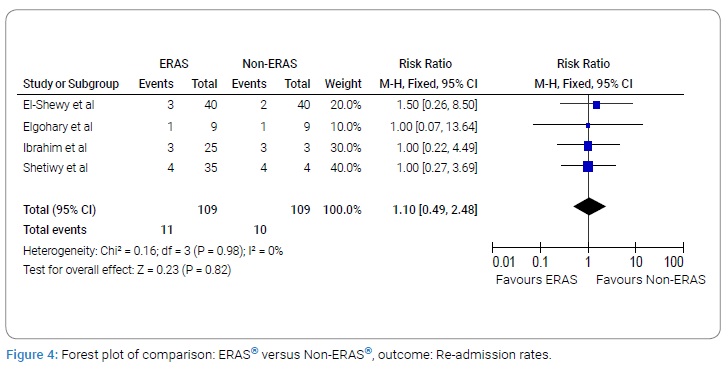
Re-admission rate
A total of four studies with 109 subjects in the ERAS® group and 109 subjects in the Non-ERAS® group provided data on Re-admission rate. Based on the I2 test (I2–0%) and Chi-squared test (P = 0.98), we chose the fixed effects model to analyze Re-admission rate due to low variability. The pooled results showed that there was no significant difference between ERAS® group and Non-ERAS® group with regards to re-admission rates (RR: 1.10, 95% CI:0.49 to 2.48, P = 0.82, (Figure 4)).
Sensitivity analysis
Using random-effects or fixed-effects models did not affect the pooled effect size in analysis of any of the reported outcomes. The direction of pooled effect size remained unchanged when OR, RR or RD was calculated for dichotomous variables. The direction of pooled effect size remained unchanged when MD or SMD were calculated for continuous variables.
Discussion
An updated and comprehensive assessment of the evidence concerning practice of Enhanced Recovery after Surgery (ERAS®) in African countries is lacking. We performed a comprehensive systematic review and meta-analysis of comparative studies to evaluate comparative outcomes of the Enhanced Recovery after Surgery (ERAS®) and Non-ERAS® in patients undergoing gastrointestinal surgeries in Africa. Our study identified eight comparative studies [14–21] reporting a total of 451 patients of whom 224 underwent gastrointestinal surgery procedures using ERAS® protocol and the remaining 227 had gastrointestinal surgery procedures done without using ERAS® protocol. The meta-analyses of outcomes showed that gastrointestinal surgery procedures using ERAS® protocol was associated with significantly shorter length of hospital stay and fewer complication rates compared to the gastrointestinal surgery procedures not using ERAS® protocol (Non-ERAS® group). However, there was no significant difference in re-admission rates between two groups. Our findings with regard to length of hospital stay, complication rates and re-admission rates are consistent with finding of a meta-analysis conducted by Lau et al. [22] in 2016. In their meta-analysis that involved Forty-two studies with 5,241 patients showed that ERAS® programs reduced Length of Stay, complication rates, and hospitalization costs across all types of surgeries. No difference in mortality or readmission rates was seen; but it was observed in their study that 30-day readmission rates following upper GI surgeries almost doubled with the application of ERAS® programs. The results of this meta-analysis provide best available evidence that practice of Enhanced Recovery after Surgery (ERAS®) Programs in African countries is feasible and safe in patients undergoing gastrointestinal surgery procedures. The adoption and full implementation of ERAS® programs in African surgical practice could offer reduction in length of hospital stay and postoperative complications at the same improving postoperative recovery to most of African surgical patients. This study has some limitations which should be considered when interpreting our findings. First, there have been few comparative studies conducted with regards to the subject, and the number of patients included in this review is not huge. Second, there was significant heterogeneity for length of hospital stay. This compelling heterogeneity may be attributable to study design of included studies (both RCT and non-RCT), clinical factors (for example, the technical skill level of the surgeon and hospital, type of gastrointestinal surgery procedure), and outcome evaluation approaches. Furthermore, the study included did not apply uniform recommendations of ERAS® implementation. There is a potential language bias in this study because of inclusion of only English-language studies. Finally, some of the included studies did not report their respective standard deviations for continuous data. We had to calculate their mean and standard deviation by applying the approach described by Hozo et al. [23] this might have brought some degree of bias.
Conclusion
Our meta-analysis (level 2 evidence) showed that the Enhanced Recovery after Surgery (ERAS®) Practices in African settings has similar advantages of shorter hospital stay and less postoperative complications as in developed countries. Progressive adoption of ERAS® in routine surgical practices would benefit many patients undergoing gastrointestinal surgery procedures in African countries.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Informed consent was obtained for this publication.
References
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606–617.
- Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322(7284):473–476.
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641.
- Kehlet H, Kennedy RH. Laparoscopic colonic surgery--mission accomplished or work in progress? Colorectal Dis. 2006;8(6):514–517.
- Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev. 2007;2007(3):CD004929.
- Feldheiser A, Aziz O, Baldini G, Cox BPBW, Fearon KCH, Feldman LS, et al. Enhanced Recovery after Surgery (ERAS®) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289–334.
- Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CHC, et al. Guidelines for perioperative care for liver surgery: Enhanced Recovery after Surgery (ERAS®) society recommendations. World J Surg. 2016;40(10):2425–2440.
- de Groot JJA, Ament SMC, Maessen JMC, Dejong CHC, Kleijnen JMP, Slangen BFM. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2016;95(4):382–395.
- Jones EL, Wainwright TW, Foster JD, Smith JRA, Middleton RG, Francis NK. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl. 2014;96(2):89–94.
- Roulin D, Donadini A, Gander S, Griesser A-C, Blanc C, Hübner M, et al. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100(8):1108–1114.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Elgohary H, Baiuomy M, Mohamed A, Hamed M, Mosaad A. Comparative study between enhanced recovery after surgery and conventional perioperative care in elective colorectal surgery. Egypt J Surg. 2017;36(2):137–144.
- Moydien MR, Oodit R, Chowdhury S, Edu S, Nicol AJ, Navsaria PH. Enhanced recovery after surgery (ERAS®) in penetrating abdominal trauma: A prospective single-center pilot study. S Afr J Surg. 2016;54(4):7–10.
- El-Shewy AH. Abdalla WM, Baghdadi MA. Enhanced recovery after surgery protocol in colorectal surgery. Egypt J Surg. 2020;39(1):94–101.
- Ibrahim AA, Moustafa RM, Moustafa AA, Rabaa SEL, Salama Y. Enhanced recovery program (ERP) versus traditional care after elective left side colorectal cancer surgery. Egypt J Hospital Med. 2018;72(8):5122–5129.
- Fathy M, Khedre MM, Nagaty MAM, Zaghloul NM. Enhanced recovery protocols versus traditional methods after resection and reanastomosis in gastrointestinal surgery in pediatric patients. Annals of Pediatric Surgery. 2018;14(4):214–217.
- Ndayizeye L, Kiswezi AK. Fast Track Surgery at the University Teaching Hospital of Kigali: A Randomized Controlled Trial Study in Abdominal Surgery. East Central Afr J Surg. 2017;22 (1).
- Shetiwy M, Fady T, Shahatto F, Setit A. Standardizing the protocols for enhanced recovery from colorectal cancer surgery: are we a step closer to ideal recovery? Ann Coloproctol. 2017;33(3):86–92.
- Tshijuke SM, Bajunirwe F, Munyarugero E. Enhanced recovery after surgery: Effect on outcome of patients undergoing laparotomy at Mbarara regional referral hospital. Clin Nutrition ESPEN. 2018;25:197.
- Lau CSM, Chamberlain RS. Enhanced Recovery after Surgery Programs Improve Patient Outcomes and Recovery: A Meta-analysis. World J Surg. 2017;41(4):899–913.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Keywords
Enhanced recovery after surgery; Multimodal perioperative care; Fast track surgery; Gastrointestinal urgery; Digestive system surgical procedures
Cite this article
Kanani GK, Mrisho JA, Zhaohui X, Xin C. Enhanced recovery after surgery (ERAS®) for gastrointestinal surgery in africa: a systematic review and meta-analysis. Clin Surg J. 2021;2(3):1–7.
Copyright
© 2021 George Kefa Kanani. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).





