Surgical Management of Primary Non-Hodgkin’s Lymphoma of Spine
* Md. Kamrul Ahsan;
Sachindra Raj Joshi;
Md. Shahidul Islam Khan;
Mohammed Abdul Awwal;
Abdullah Al Mahmud;
Md. Hamidul Haque;
-
* Md. Kamrul Ahsan: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Sachindra Raj Joshi: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Md. Shahidul Islam Khan: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Mohammed Abdul Awwal: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Abdullah Al Mahmud: Department of Orthopaedic Surgery, IBN Sina Medical College, Bangladesh.
-
Md. Hamidul Haque: Department of Orthopaedic Surgery, IBN Sina Medical College, Bangladesh.
-
Jun 08, 2021 |
-
Volume: 2 |
-
Issue: 2 |
-
Views: 5059 |
-
Downloads: 1954 |
Abstract
Background: Primary Non-Hodgkin’s Lymphoma (NHL) of the spine is an uncommon presentation of extra nodal lymphoma. Chemotherapy, radiotherapy, and biological therapy are standard treatment protocols for patients with NHL. In patients with neurologic compression due to primary NHL of spine, the role of surgical decompression still remains unclear. We retrospectively evaluate the improvement of neurological function and the survival rate following decompressive surgery of 17 NHL patients involving the spine.
Methods: Records of 8 men and 9 women aged 12 years–64 years (mean, 48.5) years, who underwent treatment for primary NHL involving spine with neurologic compression were obtained from the year 2005–2020. All patients had mild to moderate back pain and neurological deficits. Imaging features demonstrate an epidural spinal mass with involvement of vertebral body and diagnosis confirmed by histopathological examination as diffuse large B-cell non-Hodgkin’s lymphoma. All patients underwent wide laminectomy with or without posterior instrumentation along with six cycles of chemotherapy.
Results: They were followed on an average of 50.7 months (range 5 months–108 months). Following the decompressive surgery, 64.7% (n = 11) showed complete recovery to grade E, 23.5% (n = 4) recovered to grade D, 11.8% (n = 2) recovered to grade C. After surgery two patients died at 5 months and 24 months due to the disease process. The 5 years survival rate was 88.2%.
Conclusion: Surgical decompression potentially provides good local control and recovery from the neurological deficit, thus improving the overall survival rate.
Introduction
Primary Non-Hodgkin’s Lymphoma (NHL) rarely presents in the spine as extranodal lymphoma. Spinal Epidural Involvement (SEI) occurs in 0.1%–6.5% of patients with NHL, either at presentation or as a complication during the course of the disease [1–3]. The tumor tissue derives from paraspinal lymph nodes or from involved vertebral bodies, but it has been suggested that primary lymphoma may develop de novo from epidural lymphoid rest [4]. Neurological compression due to primary NHL of the spine has devastating consequences in patients. There are various alternatives of treatment for primary NHL of the spine, which include either radiotherapy or chemotherapy, or a combination of both; and also surgical excision of the tumor mass along with chemotherapy or radiotherapy. But basic guidelines for the treatment of primary NHL with neurological compression have not been defined. Chemotherapy regime following decompressive surgery for cord compression gives the best chance of local control and therefore best functional result [4]. This study aimed to evaluate the improvement of neurological function following decompressive surgery for local lesion and the overall survival rate following this surgery.
Material and Methods
We retrospectively reviewed 17 patients of primary NHL involving the spine from October, 2005 to April, 2020 after approval from the Institutional Review Board. The clinical information data were obtained from the patient’s medical records. Demographic variables, clinical presentation, neurological function, laboratory investigations; imaging features which included the primary location of the disease, presence of involved vertebrae, spinal cord or nerve root compression, and metastasis as well as staging; pathological diagnosis, treatment modality, and last follow-up were also obtained (Table 1).
All patients had mild (n = 10) to moderate (n = 7) back pain and neurological deficits. Multiple variables were studied, including; neurological deficits and patient survival outcomes.
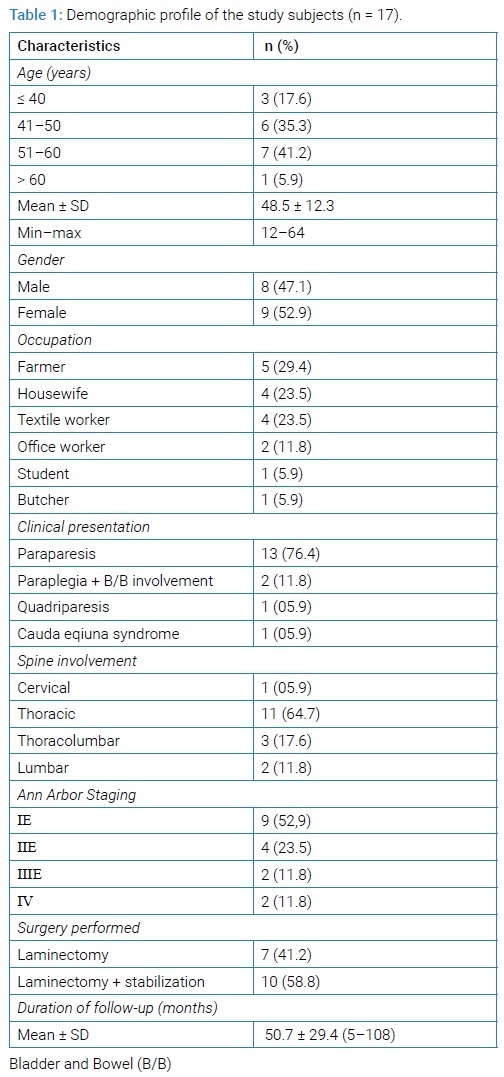
Diagnostic workup: All patients underwent radiological assessment plus laboratory investigations. The radiological investigation incorporated plain X-ray of the spine (anteroposterior & lateral view), MRI of the primary site, chest X-ray, Computed Tomography (CT) of the chest, abdomen & pelvis; and ultrasound of whole abdomen and pelvic organ. Bone scan was performed in almost all the patients. Radiological features of primary NHL involving spine were usually normal; sometimes lytic, mixed lytic/ sclerotic, or sclerotic lesions were seen. T1-weighted MRI images showed low signal intensity and T2-weighted images showed a high signal intensity of the lesion due to bone narrow infiltration and also revealed extra-osseous soft tissue mass surrounding the cord (Figure 1A and B). Contrast-enhanced MRI also showed extensively enhancing hypointense marrow disease with extra-osseous soft tissue (Figure 1C).
Laboratory investigations included Complete Blood Count (CBC), C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), Hematocrit (Hct), serum protein electrophoresis, Lactate Dehydrogenase (LDH), uric acid, renal and liver function test, and urinalysis. Pathological diagnosis was done with Computed Tomography guided Fine Needle Aspiration Cytology (CT FNAC) and/ post-operative biopsy as well as bone marrow aspiration study.
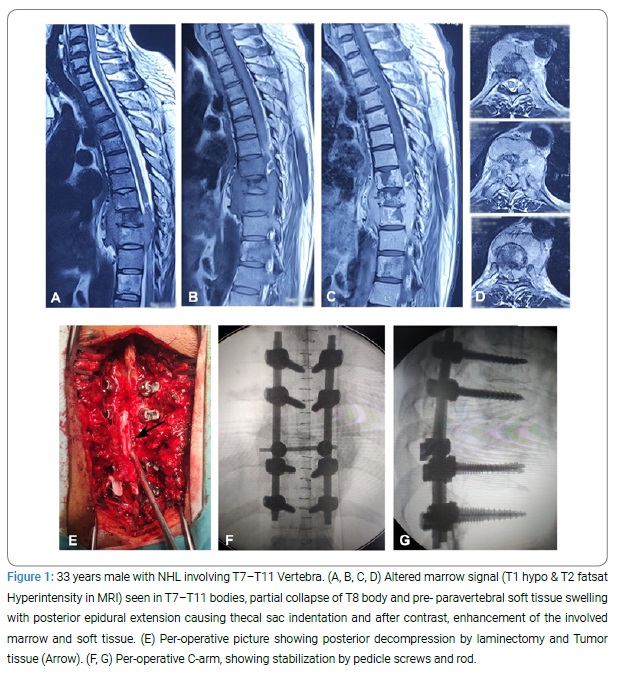
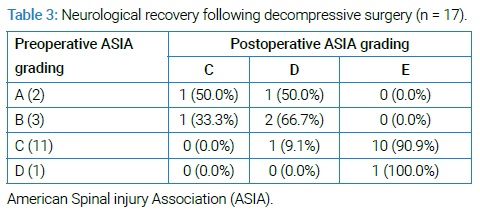
Patient Staging & Neurological Evaluation: Patients were staged according to the Ann Arbor staging classification. They were categorized as stage IE (single localized spine lesion), stage IIE (single spinal lesion and adjoining or closely associated lymph nodes), stage IIIE (distant nodal involvement), and stage IV (diffuse involvement of spine and organ or sites) (Table 1). We evaluated the neurological impairment and recovery after surgery according to the American Spinal Injury Association (ASIA) impairment scale (Table 2 and 3).
Treatment: All patients underwent surgical decompression by laminectomy with or without stabilization under general anesthesia (Table 1 and Figure 1). After laminectomy, we try to remove all the epidural soft tissue mass that encircle the spinal cord and nerve root and then stabilized the spine where indicated. All excised specimens were sent for histopathological examination for confirmation of diagnosis. Post-operatively all patients underwent chemotherapy 3-weekly cyclophosphamide (750 mg/m2, IV, day 1), hydroxydaunorubicin /doxorubicin (50 mg/m2–75 mg/m2, IV, day 1), vincristine (1.4 mg/m2–2 mg/m2, IV, day 1), prednisolone (40 mg/day–100 mg/day, day 1–5) [CHOP] regimen, for up to 6 cycles. One patient with CD20 positive NHL had an R-CHOP regimen (R-rituximab 375 mg/m2, IV, day 1+ CHOP therapy).
Follow-up evaluation: After treatment, we evaluated the patients by careful history taking and physical examination. In addition, we also performed regular laboratory investigations at every follow-up visit which include CBC and serum chemistries, including lactate dehydrogenase and other blood tests; and imaging studies for relevant clinical indications. Imaging studies include positron emission tomography (PET)/CT with [18F] fluorodeoxyglucose (FDG) and MRI. According to revised International Working Group Response Criteria [5], we see the changes in size and FDG avidity of the tumor by PET/CT with FDG and in MRI we also evaluated the tumor signal intensity on T2 weighted sequences as well as in contrast enhancement. Patients were followed up 3 monthly for 2 years, 6 monthly for 3 years, and then annually for at least 5 years after completion of treatment [6].
Statistical analysis: The quantitative data were analyzed statistically using Statistical Package for the Social Science (SPSS Inc, version 22, Chicago, IL). Statistical significance was set at p < 0.05 and confidence interval set at 95% level. The continuous variable was expressed as mean with standard deviation and categorical variables as the frequency with percentage. Numerical data were assessed by paired t-test.
Results
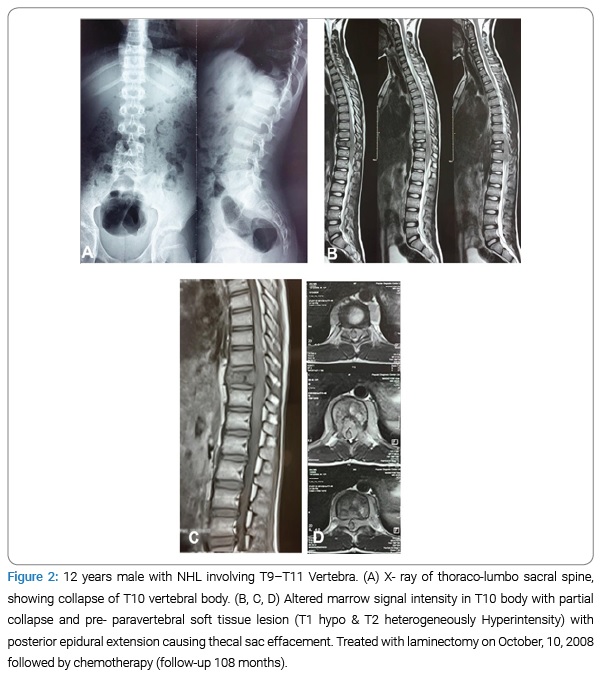
Patient demographics and presenting characteristics: Patients averaged 48.5 years of age (range, 12 years–64 years) and the mean follow-up period was 50.7 (range, 5 months–108 months). Most involved region was thoracic [n = 11 (64.7%)] region followed by thoracolumbar [n = 3 (17.6%)], lumbar [n = 2 (11.8%)] and cervical region [n = 1 (5.9%)] (Table 1). Most of the patients presented with paraparesis [n = 11 (64.7%)] followed by paraplegia with bowel and bladder involvement in [n = 3 (17.6%)], other patients presented with quadriparesis [n = 1 (5.9%)], and cauda equine syndrome [n = 1 (5.9%)]. Neurologic symptoms were mainly caused by soft tissue compression in 7 patients, and soft tissue compression along with vertebral fracture with instability in 10 patients (Table 4 and Figure 1 and 2A–2C).
Clinical staging and neurological assessment: All patients had a histopathological diagnosis by either CT guided FNAC (further confirmed postoperatively) [n = 8 (47.1%)] or postoperative biopsy [n = 9 (52.9%)]. According to the Ann Arbor Staging system 9 patients (52.9%) were at IE stage, 4 patients (23.5%) were at IIE and 2 patients (11.8%), each in IIIE and IV stage. At the time of presentation, most of the patients were at ASIA grade C [n = 11(64.7%)] followed by grade B [n = 3(17.6%)], grade A [n = 2 (11.5%)] & grade D [n = 1(5.9%)]. Following the decompressive surgery, 64.7% (n = 11) showed complete recovery to grade E, 23.5% (n = 4) recovered to grade D, 11.8% (n = 2) recovered to grade C (Table 3). No major complications were observed during treatment except superficial wound infection in 2 patients which were managed conservatively and chemotherapy related complications occurred in 5 patients which included hepatitis [n = 1(5.8%)], renal impairment (increased serum urea and creatinine) [n = 2 (11.8%)] and hyperuricemia [n = 1(5.8%)], phlebitis (I/V cannula site) [n = 1(5.8%)] and all chemotherapy related complications gradually improved after adjustment of drug regimen over a period of 12 weeks.
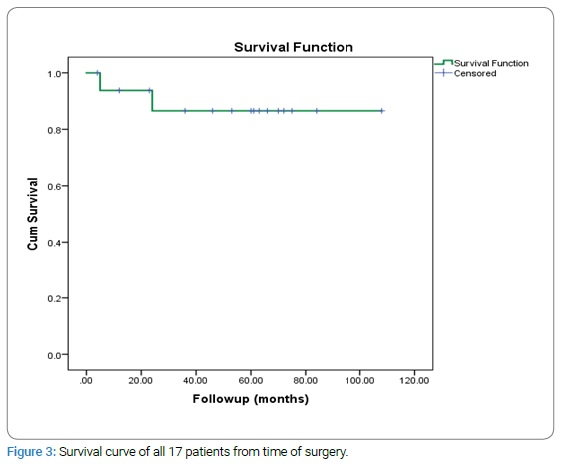
Survival analysis: Fifteen patients were alive at the latest follow-up and 2 patients died at 5 months and 24 months follow-up after surgery due to the disease process. The 5 years overall survival rate was 88.2% as shown in the survival plot (Figure 3).
Discussion
Non-Hodgkin Lymphoma (NHL) is a monoclonal proliferation of lymphoid cells of B-cell (90%) or T-cell (10%) origin, widely disseminated at presentation, including in extranodal sites [7,8]. The incidence of primary non-Hodgkin’s lymphoma of the bone (PNHLB) is 7% of all malignant bone tumors, 4%–5% of all extra- nodal NHL, and less than 1% of all malignant lymphomas [9]. Spinal presentations of NHL have been described in all age groups; however, mostly presents after 4th decade of life with almost equal male-female prevalence [3,4,10]. In our series, patients clustered around 4th and 5th decade and had slightly female predominance. Boffetta and Vocht [11], reported in their study that those who work in the printing industry, woodworkers, farmers, and teachers are more vulnerable to NHL; which was consistent with our study (Table 1).
Various authors stated that primary NHL of the spine rarely causes neurologic compression (0.1%) while others reported 3.3% to 5% neurologic compression [10,12], while in our study, almost all the patients presented with some degree of neurological deficit. Most of them presented with paraparesis (ASIA Grade C) while few patients presented with, paraplegia with bowel and bladder involvement, which were consistent with other studies [3,13–15]. NHL commonly involved the thoracic region of the spine because of the greater length of the thoracic vertebra in relation to others and the particular lymphatic drainage of the vertebral column [3]. In our series thoracic region was the most common site for the disease followed by lumbar region and rarely in cervical region which was consistent in other case series [8,13–15]. Diagnosis of the NHL of spine is often missed or delayed due to lack of specific findings and presentation that mimics with other diseases like small round cell neoplasm (multiple myeloma and Ewing sarcoma), metastasis and tubercular infection; hence diagnosis is always made by histopathological examination [16]. In our study, we opted for CT FNAC, where many of times results were inconclusive as was seen in study done by Acharya et al. [13] In our study almost all the patients had a neurological deficit and they underwent decompressive surgery where biopsy was obtained and then further diagnosis was ensured. Though NHL is effectively treated with chemotherapy, radiotherapy, biological therapy, and hematopoietic stem cell transplantation but surgery may be useful for removing the local disease as well as confirming an equivocal radiological diagnosis through biopsy. The proper management and prognosis of patients presenting with spinal epidural lymphomais not yet clear because of the lack of homogeneity in the selection of patients. Literature review showed chemotherapy and/or irradiation following surgical excision of the tumor, as the treatment of choice for neurologic compression due to primary NHL of the spine [15]. Studies done by Epelbaum et al. [1] and Eeles et al. [4] showed good recovery of neurological deficit after decompressive surgery. Another study by Chang et al. [14] showed, recovery rates from neurological deficit were better in the operative group in compared to the non-operative group and on the other hand, Peng et al. [15] showed in their study that the ideal treatment for neurologic compression by soft tissue extension in primary NHL of spine, was combination of chemotherapy and radiotherapy; irrespective of the surgical treatment. In our study complete recovery was seen in 64.7% (Grade E), 23.5% recovered to grade D, 11.8% recovered to grade C, in combination with chemotherapy (CHOP) following surgery. Patients with an initial grade of C or D had a greater chance of complete recovery following surgery than patients with an initial grade of A or B (Table 3). Early and good recovery from neurological deficit following decompressive surgery is considered for local control of disease and avoiding further damage to spinal cord. Chemotherapy or radiotherapy following surgery didn’t affect the further neurological status [2,4]. However, the fundamental goal is complete recovery from neurological deficit and complete remission of the disease process, so as to improve quality of living. As it was difficult to completely resect the local lesions, the main role of surgery was to alleviate the spinal compression, obtain biopsy and spinal stabilization; whereas chemotherapy was instituted for primary treatment of disease process for its complete remission. The combined treatment modality had increased the overall survival rate in Primary NHL of spine with neurological compression [15]. As seen in our study, where overall 5 years survival rate was 88.2%. Moreover, decrease in comorbidities associated with spinal cord compression, such as cardiopulmonary dysfunctions, poor infection control; psychological problems could play an important role in superior survival observed in a patient who underwent surgical decompression [14]. It is recommended for surgical decompression in all the patients with features of spinal cord compression in Primary NHL of spine, for early recovery from neurological deficit and avoidance of its consequences and combined with chemotherapy for improving overall survival.
Conclusion
Primary NHL of the spine is a rare presentation, which mimic with other diseases of spine hence requires prompt and proper diagnosis. Surgical decompression potentially provides good local control and recovery from the neurological deficit, thus improving the overall survival rate.
Acknowledgments
The authors thank and grateful to Chairman, Department of Haemato-Oncology, BSMMU, for his cordial support during treatment.
References
- Epelbaum R, Haim N, Ben‐Shahar M, Ben‐Arie Y, Feinsod M, Cohen Y. Non-Hodgkin’s lymphoma presenting with spinal epidural involvement. Cancer. 1986;58(9):2120–2124.
- Perry JR, Deodhare SS, Bilbao JM, Murray D, Muller P. The significance of spinal cord compression as the initial manifestation of lymphoma. Neurosurgery. 1993;32(2):157–162.
- Salvati M, Cervoni L, Artico M, Raco A, Ciappetta P, Delfini R. Primary spinal epidural non-Hodgkin’s lymphomas: a clinical study. Surg Neurol. 1996;46(4):339–343.
- Eeles RA, O’Brien P, Horwich A, Brada M. Non-Hodgkin’s lymphoma presenting with extradural spinal cord compression: functional outcome and survival. Br J Cancer. 1991;63(1):126–129.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586.
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244.
- Ultmann JE, DeVita VT., Jr . Hodgkin’s disease and other lymphomas. In: Petersdorf RG, Adams RD, Braunwald E, Isselbacher KJ, Martin JB, Wilson JD, editors. Harrison’s Principles of Internal Medicine. 10th Ed. New York: McGraw-Hill; 1983. pp. 811–825.
- Watson HG, Culligan DJ, Manson LM. Haematology and transfusion medicine. In: Ralston SH, Penman ID, Strachan MW, Hobson R, editors. Davidson’s Principles and Practice of Medicine. 23rd ed. Edinburg, London: Elsevier Health Sciences; 2018;964–966.
- Unni KK, Hogendoorn PCW: Malignant Lymphoma. In: World health organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Fletcher CDM, Uni KK, Mertens F. (Eds): IARC Press. Lyon, 2002: 306–308.
- McDonald AC, Nicoll JA, Rampling RP. Non-Hodgkin’s lymphoma presenting with spinal cord compression; a clinicopathological review of 25 cases. Eur J Cancer. 2000;36(2):207–213.
- Boffetta P, de Vocht F. Occupation and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2007;16(3):369–372.
- Vanneuville B, Janssens A, Lemmerling M, De Vlam K, Mielants H, Veys EM. Non-Hodgkin’s lymphoma presenting with spinal involvement. Ann Rheum Dis. 2000;59(1):12–14.
- Acharya S, Kumar M, Adsul N, Shahi P, Chahal RS, Kalra KL. A comparative study of interventions in spinal extranodal Non-Hodgkin’s lymphoma: A single-center case series from North India. Indian Journal of Med Special. 2020;11(4):201–206.
- Chang CM, Chen HC, Yang Y, Wang RC, Hwang WL, Teng CL. Surgical decompression improves recovery from neurological deficit and may provide a survival benefit in patients with diffuse large B-cell lymphoma-associated spinal cord compression: a case-series study. World J Surg Oncol. 2013;11:90.
- Peng X, Wan Y, Chen Y, Chen L, He A, Liao W, et al. Primary non-Hodgkin’s lymphoma of the spine with neurologic compression treated by radiotherapy and chemotherapy alone or combined with surgical decompression. Oncol Rep. 2009;21(5):1269–1275.
- Akgül T, Bilgin Y, Karademir G. The great mimicker at thoracolumbar spine: Non-Hodgkin’s lymphoma. Int J Surg Case Rep. 2017;39:267–270.
Keywords
Primary non-Hodgkin’s lymphoma; Spine; Neurological deficit; Decompressive surgery; Chemotherapy
Cite this article
Ahsan MK, Joshi SR, Khan SI, Awwal MA, Mahmud AA, Haque MH. Surgical management of primary non-hodgkin’s lymphoma of spine. Clin Surg J. 2021;2(2):1–7.
Copyright
© 2021 Ahsan MK. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).







