Surgical Management of Adult High-Grade Spondylolisthesis by Partial Reduction, Decompression and Instrumented Circumferential Fusion
* MD. Kamrul Ahsan;
Krishna Kant Yadav;
Md. Shahidul Islam Khan;
Mohammed Abdul Awwal;
Sachindra Raj Joshi;
Abdullah Al Mamud;
Md. Hamidul Haque;
-
* MD. Kamrul Ahsan: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Krishna Kant Yadav: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Md. Shahidul Islam Khan: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Mohammed Abdul Awwal: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Sachindra Raj Joshi: Department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University (BSMMU), Bangladesh.
-
Abdullah Al Mamud: Department of Orthopaedic Surgery, IBN Sina Medical College, Bangladesh.
-
Md. Hamidul Haque: Department of Orthopaedic Surgery, IBN Sina Medical College, Bangladesh.
-
Apr 22, 2021 |
-
Volume: 2 |
-
Issue: 2 |
-
Views: 7098 |
-
Downloads: 2365 |
Abstract
Background: Treatment options of symptomatic High-Grade Spondylolisthesis (HGS) are surgery. But surgical treatment of HGS is often difficult and related techniques are still debated.
Objectives: To assess the outcome of adult HGS by partial reduction, instrumentation and circumferential fusion in a single institution.
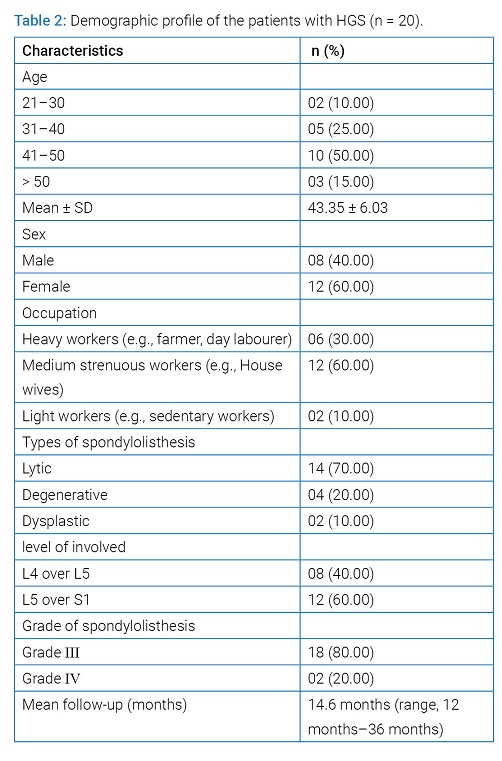
Materials and Methods: This quasi experimental study was carried out on 20 patients of HGS with various etiologies (lytic 14, degenerative 4 and dysplastic 2) with mean age 43.35 years (range, 21 years to 55 years). Diagnosis was confirmed by history, clinical examination and radiology. Patients were evaluated clinically by VAS scale, functionally by Oswestry Disability Index (ODI), radiological fusion by Hackenberg criteria and overall outcome by Inoue’s criteria.
Results: The average follow-up was 14.6 months. All patient had low back pain(n = 20), radiculopathy and claudication pain (n = 16), cosmetic deformity (n = 4), palpable step (n = 20), hamstring tightness (n = 8), SLR test positive (n = 6), sensory involvement (n = 16) and motor involvement in (n = 14) patients.Level of involvement was at L5–S1(n = 14) and L4–L5 (n = 8). There were 18 Meyerding’s Grade III and 2 Grade IV slips. Amazing changes were observed in percentage of slip, slip angle, disc height and spino-pelvic parameters after 12 months of operation. Mean VAS reduced from 6.80 to 2.00 and mean ODI reduced from 67.5 to 7.00. There were no pseudarthroses or significant instrumentation failures. Fusion achieved in 20 (100%) cases. Seventeen (85%) patients got satisfactory outcome.
Conclusions: Partial reduction, decompression and instrumented circumferential fusion is a viable option in adult High-Grade slip for relieving the symptoms, achieving stability and fusion.
Introduction
Spondylolisthesis a common spinal condition in adolescents and adults defined as displacement of one vertebral body over another, was first used by Killian [1] in 1854 but the pathology was first noted in 1772 by Herbiniaux [2], a Belgian obstetrician. Translation more than 50% are defined as High-Grade Spondylolisthesis (HGS) which include as per Meyerding’s [3] classification grade III (51%–75%), grade IV (76%–100%) and grade V (> 100% slip) termed as spondyloptosis [4] and are generally associated with a higher risk of slip progression and disabling symptomatology. Most adult HGS are isthmic in nature and involve at L5–S1 level. The incidence of spondylolisthesis in the general population is 4%–8% [5], whereas HGS is found approximately 1% in those with spondylolisthesis patients [6]. Patients with HGS are frequently complaints of low back pain, leg pain from nerve root irritation, most commonly the L5 root and associated with postural deformities such as compensatory lumbar hyperlordosis for focal kyphosis, neurogenic claudication, motor weakness and occasionally bowel and bladder incontinence. The management of adult HGS remains controversial and differs from that of low-grade slips [7,8]. Surgical intervention for symptomatic HGS is more favorable than non-operative management to halt progression of deformity and as well as provide symptom relief. Various options for surgical treatment of HGS includes Gill procedure, reduction and fusion, decompression with insitu fusion and Gaines procedure (L5 vertebrectomy and reduction of L4 to S1 and fusion) [9–13]. There are numerous classifications for spondylolisthesis but recently Spine Deformity Study Group (SDSG) [14] classify spondylolisthesis on the basis of slip grade and spino-pelvic alignment as low grade (< 50%) or high grade (> 50%). High grade again divided into 3 more types: type IV (balanced pelvis), type V (retroverted pelvis with balanced spine), and type VI (retroverted pelvis with unbalanced spine) and offers clinical guidelines to the surgeon for treatment options and also assess the need for reduction of the slip [15,16]. The role of reduction of adult HGS is still debatable [17–22]. Patients with HGS usually associated with sagittal spinopelvic morphotype which entailing a predisposition for slipping: elevated Pelvic Tilt (PT) associated with elevated Sacral Slope (SS), Pelvic Incidence (PI) and Lumbar Lordosis (LL) [23]. Reduction should be considered when there is evidence of segmental instability or segmental sagittal imbalance is present [24,25] which improved biomechanics, higher fusion rates and cosmetic correction of deformity [17,26,27] but associated with significant risk of complications including neurological deficit [5,21,28,29]. On the other hand, in-situ fusion minimizes neurologic complications but associated with risk of slip progression, pseudoarthrosis and persistence of cosmetic deformity [10,18,30,31]. Despite the existing debate in the literature, it can be extrapolated from experience with sagittal imbalance disorders that at least partial reduction and correction of the underlying lumbosacral kyphosis should be achieved whenever possible, as long as the operative risk are acceptable [23]. The Posterior Lumbar Interbody Fusion (PLIF) procedure is biomechanically stronger than Posterolateral Fusion (PLF) procedure and provide axial support with less graft subsidence or collapse and allow a better biologic fusion in lordotic alignment with some reduction [32,33]. Although there is absence of high-quality evidence to enable definitive recommendations. Moreover, there are a limited number of adult High-Grade studies to aid the surgeon in sound decision making. The objective of the study is to analyzes the outcome of 20 patients of adult symptomatic HGS who underwent partial reduction, instrumentation and circumferential fusion (PLIF + PLF) in a single institution.
Material and Methods
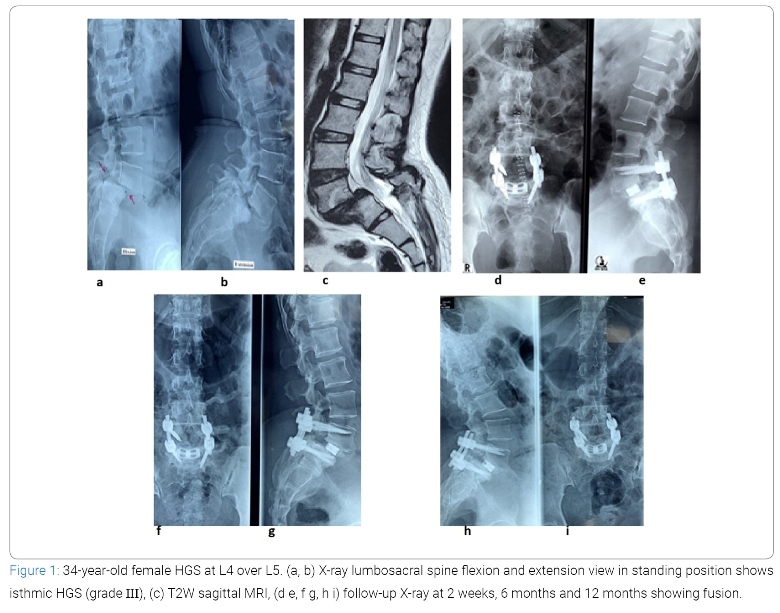
This quasi-experimental study was carried out in our Orthopaedic dept, from January 2016 to January 2020. Total 20 patients with HGS were purposively selected according to selection criteria. After obtaining Institutional Review Board (Ref.No.6615) permission, we are explaining the study objectives, purpose and potential risks of the procedure in details to the patients or patient’s attendance before their inclusion. Included criteria were: (1) back pain with diagnosed HGS with or without neurological deficit; (2) failure of conservative management; (3) progression of subluxation; (4) global sagittal imbalance (5) progressive motor deficit, or cauda equina syndrome; (6) spondylolisthesis involving L4 over L5 and L5 over S1. Excluded were (1) patients with HGS associated with infection and malignancy. (2) patients with severe osteoporosis, (3) patients who are not operable from medical ground and (4) less than 12 months of follow-up. Records of 8 men and 12 women aged 21 years to 55 years (mean, 43.35 years) who underwent surgical treatment for HGS with various etiologies (lytic 14, degenerative 4, dysplastic 2) at L4 over L5 (n = 8) and L5 over S1 (n = 12) level were reviewed. According to Meyerding’s classification, 18 cases had grade III and 2 case had grade IV spondylolisthesis. The patients were diagnosed by history and physical examination, supported by appropriate radiological investigations which includes X-ray of whole spine in standing position antero-posterior and lateral view, dynamic X-ray of the lumbosacral spine as well as Computed Tomography (CT) scan and Magnetic Resonance Imaging (MRI). Whole spine radiographs show the spino-pelvic parameters (PI, PT, SS) and the global spinal sagittal balance and dynamic films are required to see the end-plate irregularities, defects in the subchondral bone, sclerosis, vertebral collapse and instability at the involved segment. Antero-posterior, lateral and oblique projections in lumbar spine are required to show the typical appearance of an Inverted Napoleon Hat, evidence of dysplasia of the posterior elements, pars defects and to document the grade of slip as per Meyerding’s grade (Figure 1a and b). CT scan is required to see the dimensions of the L5 pedicle on the axial sections and also confirm the presence of pars defects. MRI is done to see the severity of involvement of central canal, lateral recess and neural foramen and also helps to evaluate the status of the adjacent disc level and determined the ultimate level of superior extent of fusion (Figure 1c). Detailed history, clinical examination findings and all information were taken in a predesigned duly pretested data collection form. Demographic variables, clinical variables, hospital stay and post-operative complications were recorded. Pre-operative and post-operative clinical assessment were done by using VAS scale for pain relief and ODI score [34] for improvement of disability. All pre-operative and post-operative radiological findings (e.g., percentage of slip, disc height, slip angle, spino-pelvic parameters), types of spondylolisthesis, complications from the instrumentation and fusion status were also recorded and observed (Figure 1d–i).
Various radiographic parameters used to define spondylolisthesis and sagittal spinopelvic alignment are illustrated in (Figure 2A and B).
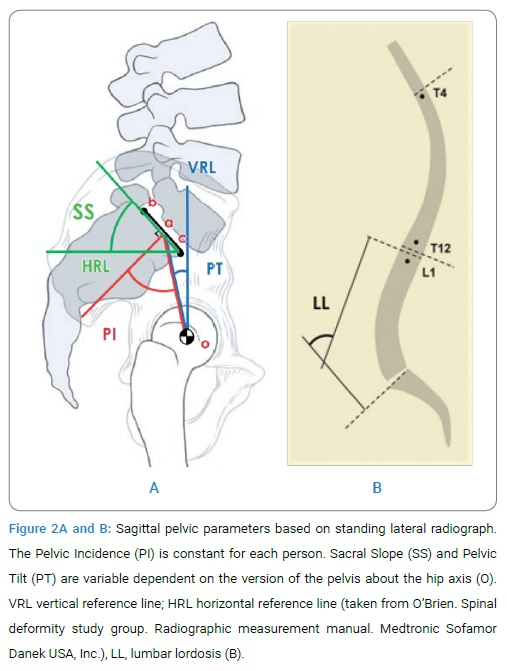
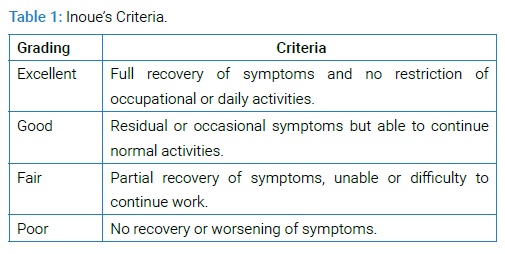
Percentage of slip as per Meyerding classification [3], grade III and IV are recognized as high grade slip and disc height may be defined as space between inferior end plate of superior vertebrae and superior end plate of lower adjacent vertebrae and slip angle is an angle between superior end plate of lower and inferior end plate of upper adjacent vertebrae [35]. SS is defined as the angle subtended by a Horizontal Reference Line (HRL) and the sacral end-plate line (Figure 2A) and normal values range from 19.50 to 65.50 with a mean value of 39.40 [36]. PT is defined as the angle subtended by a Vertical Reference Line (VRL) originating from the center of the femoral head (o) and the midpoint of the sacral end plate (a) (Figure 2A). It is positive when the hip axis lies in front of the middle of the sacral plate andnormal values range from -10 to 27.90 with a mean value of 12.30 [36]. PI is defined as an angle subtended by a line that is drawn from the center of the femoral head to the midpoint of the sacral end plate (oa) and a line perpendicular to the center of the sacral end plate (a) (Figure 2A). PI is the sum of SS and PT and normal values range from 330 to 850 with a mean value of 51.70 [36] and LL is the Cobb angle measured from the superior endplate of L1 to the superior endplate of S1 [4] (Figure 2B) and normal LL values range between 260 and 760 with a mean of 46.50 [36]. All patients were operated by single spine surgeon and were followed up at 1 month, 3 months, 6 months, 12 months consequently and yearly thereafter with X-ray and CT scan or MRI if needed. Achievement of fusion assessed by criteria of Hackenberg et al. [37] by two independent observers. Overall comprehensive outcome of each patent was assessed by Inoue’s criteria [38] (Table 1).
All the data were checked and edited after collection. Then the data was entered into computer and statistical analysis of the results was obtained by using windows-based computer software Statistical Packages for Social Science (SPSS Inc, version 22, Chicago, IL). Statistically significant was set at p < 0.05 and confidence interval set at 95% level. Continuous variables were expressed as mean with standard deviation and categorical variables as frequency with percentage. Categorical data were assessed by Chi-square test and numerical data were assessed by paired Student t-test.
Surgical procedure
After giving general anaesthesia, a foleys catheter is inserted to prevent distension of bladder and then patient was positioned in prone position on a spinal frame with abdomen free from external pressure to ensure minimal epidural bleeding and maintenance of lumbar lordosis. Perioperative antibiotics are administered prior to skin incision. After taking strict aseptic precaution, posterior midline incision was made which was 12 cm–15 cm long. Paraspinal muscle was detached subperiosteally on both sides upto the middle of the transverse process. The facet joints of the involved segments, pars interarticularis and transverse processes are identified. Meticulous control of hemostasis was done at each stage which can substantially reduce blood loss. After identification of entry point of the pedicle, pedicle screws (Universal company, India) are inserted using a standard “free hand targeting” technique under fluoroscopic control. Reduction techniques were done as described by Ruf et al. [10] and Shufflebarger et al. [31]. According to involved level (L4 over L5 or L5 over S1), a temporary screw placement is done either in L3 or L4 to achieve distraction and partial reduction of L4 or L5. Further reduction of slip and kyphotic angle was done after a wide laminectomy, adequate decompression of L4 or L5 roots as well as discectomy and interbody space preparation. If involved level at L5–S1 space, pedicle screws should then be placed at L4 and S1 level and reduction pedicle screws at L5 level. A temporary rod is place within the screw heads and distraction created L4 (or L3 if used) and S1 to aid with reduction of L5 and to open the L5–S1 disc space. Nerve root retractors are used to protect and retract the dura and traversing nerve root medially thereby exposing the disc space. Using a 15 or 11 blade, the box annulotomy is performed, disc fragments can be loosened and removed with a combination of curettes, disc space shavers, kerrison punches and pituitary rongeurs. In addition, disc space distraction is done via pedicle screw instrumentation or disc space distractors for removing disc material and adequate preparation of the endplates by intradiscal shavers with side cutting flutes. The same procedure is done bilaterally on both sides of the thecal sac. Then a cage sizer (template) is used to determine the size of the titanium banana cage (universal company, India). Two sizable rods are cut, contouring and applied to the pedicle screw heads with application of nuts. Reduction of slip is then carried out by reduction pedicle screws in advancing locking caps into the heads of reduction screws slowly and symmetrically. The reduction itself should be conducted slowly under fluoroscopic guidance. Allow time for soft tissues to relax and close communication should be maintained with the neuromonitor staff so that any changes in EMG are noted immediately. Then prepared Autogenous Graft (AG) from the excised spinous process and lamina is placed into the disc space. After removing the rod on one side, the appropriate size of titanium banana cage packed with cancellous AG, are inserted in the prepared interbody space. The final position of the cages is confirmed fluoroscopically. A visual check ensures that there is no persistent nerve root compression following the insertion of cage. The neural foramen is check and adequate trimming is done to ensure decompression. Before final tightening of the nuts, the segments are gently compressed through transpedicular instrumentation. Remaining AG grafts are applied to the prepared postero-lateral decorticated beds. Then applied spongiostum for haemostasis and for exposed dura and nerve roots. After proper haemostasis wound is closed in layers with a drain in situ. Day after operation patients are ambulating with assistance and physical therapy beginning and also advised to use lumbar corset for at least 6 weeks.
Results
The average follow-up was 14.6 months (range, 12 months–36 months). All patient had low back pain that worsened with physical exertion (n = 20), radiculopathy and claudication pain (n = 16), cosmetic deformity (n = 4), palpable step (n = 20), hamstring tightness (n = 8), SLR test positive (n = 6), Sensory involvement (n = 16) and motor involvement in (n = 14) patients. 60% involvement was at L5–S1 level and 40% at L4–L5 level. The average percentage of slip was 58.75 (± 5.56) %, 80% cases were grade III (51%–75%), and 20% were in grade IV (76-100 %). Heavy workers were 6 (30%), medium strenuous workers 12 (60%) and light workers 4 (10%) patients (Table 2).
All patients had a reduction of their High-Grade slip as part of their surgical procedure. Out of 20 patients, partial reduction was done in eighteen patients and complete reduction was done in two patients. Mean operative time was 145 minutes (range, 135 minutes–180 minutes), average blood loss during operation was 275 ml (range, 250 ml–450 ml), average length of hospitalization was 14 days (range, 10 days–21 days) and need for blood transfusion was required in 12 patients. Amazing changes was observed in percentage of slip, slip angle, disc height and spino-pelvic parameters (SS, PT, PI and LL) after 12 months of operation (Table 3).
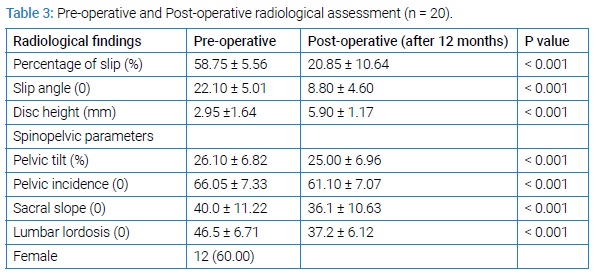
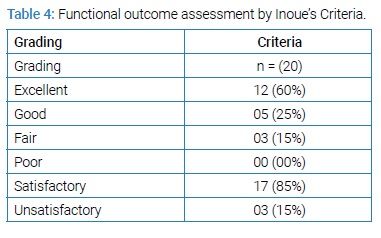
Mean VAS significantly reduced from 6.80 to 2.00 and mean ODI reduced from 67.5 to 7.00 (P value < 0.001) at 12 months follow-up. All 20 patients showed improvement in back pain and radicular pain except four patients who had mild lower back pain related to physical exertion and used NSAIDs (Non-steroidal anti-inflammatory drugs) sporadically and physiotherapy. Motor and sensory functions of all affected patients were improved. One patient out of 20, developed neurological deficit in the post-operative period which was temporary in nature and improved completely during 6 months follow-up. Dural tear occurred in two patients intraoperatively and was repaired with a good outcome. Post-operative wound infection occurred in one patient who was managed conservatively by removal of stiches, regular dressing and secondary wound closure. No pseudoarthrosis was noted. All 20 patients were monitored by X-ray and computed tomography where needed after 12 months. Fusion achieved in 20 (100%) cases. Seventeen (85%) patients got satisfactory outcome (Table 4).
Discussion
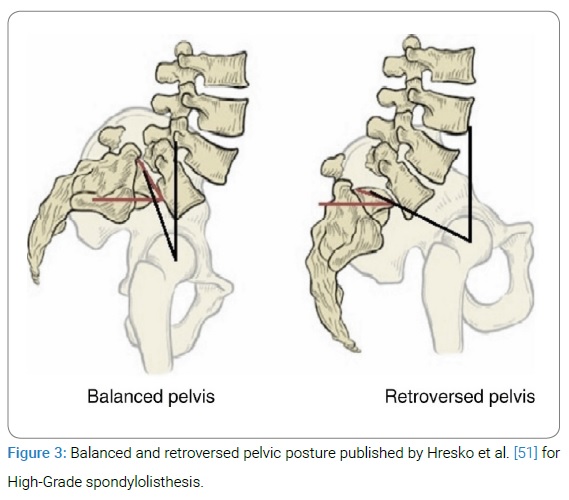
Adult HGS is a very debilitating clinical condition that carries considerable morbidity. Optimal management of HGS is complex and still remains challenging. Although there is general consensus on the need for surgical treatment of symptomatic patients presenting with severe pain, neurologic deficits, or progressive deformity but the surgical techniques remain controversial. The objectives of the treatment include resolution of lower back pain and improvement of neurological symptoms associated with arthrodesis of the affected levels and maintain of sagittal balance [39,40].The most common current surgical techniques for achieving these objectives include (1) in situ fusion techniques using posterolateral, anterior, or circumferential approaches; (2) fusion and reduction (partial or complete) combination techniques; and (3) vertebrectomy [13,39–43]. All are combined with a posterior decompression based on neurological status and presenting symptoms. But in-situ fusion, there is chance of slip progression, pseudoarthrosis formation and persisting of cosmetic deformity as well as [10,18,30,31] and in complete reduction technique, risk of neurological deficit is more that occurs during the maneuver of terminal 50% of slip reduction [5,21,44]. By this observation partial reduction with circumferential fusion is advocated by some authors [40] which can minimize neurological deficit by avoiding significant stretch of the L5 and L4 nerve roots. Recent advances in spinal instrumentation, improved understanding of the pelvic anatomy and its role in determining sagittal spinopelvic alignment, and its influence on the development of HGS have had a significant impact on surgical management of HGS. Although not proven in randomized studies, posterior instrumentation and fusion with attempted partial deformity reduction and interbody structural support have been gaining widespread acceptance and shown to provide satisfactory rates of fusion and a good clinical outcome. Regardless of the choice of surgical technique, significant complications can be associated with the surgical treatment of HGS which may dictate the type of surgical approach chosen. Age is an important factor for management of HGS. The younger the patient, the greater the risk of progression, because the deformity is likely to progress during the periods of active spinal growth [4]. Therefore, HGS in children and adolescents are more prone to surgery than in adult slips. Asymptomatic adult HGS can be observed, which is not true in children and adolescent’s patients. On the other hand, patients with adult High-Grade slips who have pain or radicular symptoms and not responding to conservative treatment are needed surgery.50% of the patients in this study, were in age group within 41 years–50 years (range, 21 years–55 years) which are comparable to the other study [17,45]. The most commonly affecting level of involvement by HGS in adults is at L5–S1 vertebral segment, rarely at L4–L5 level [6,17] and most often they are isthmic or dysplastic in nature [17,25,40] which was almost similar to our study. The exact pathophysiology of development of HGS is not well known but there is strong evidence of familial association. Approximately 25% to 30% of first-degree or second-degree relatives are associated with HGS [46]. In one study showed that there is evidence of familial incidence 33% in case of dysplastic spondylolisthesis and 15% in case of isthmic variant with a multifactorial autosomal dominant pattern of inheritance with incomplete penetrance [47]. PI is an important anatomic parameter and a strong determinant of pelvic orientation and lumbar lordosis i.e., the higher the PI, the greater will be the PTor SS, or both and thus, of sagittal spino-pelvic alignment [48,49]. Many publications reported that there is an association between PI and spondylolisthesis and noted that increased PI could predispose to spondylolisthesis [15,49,50]. After analyzing the patients with HGS, recently Hresko et al. [51] have reported two distinct groups namely ‘‘balanced (high SS / low PT)’’ and ‘‘unbalanced (low SS / high PT)’’ pelvis (Figure 3) and showed that patients with an unbalanced pelvis had a sagittal spinal alignment and required reduction techniques that differed from those with a balanced pelvis.
Another study proposed that reduction techniques should be preferable in order to restore global spino-pelvic balance and improve the biomechanical environment for fusion [52]. Labelle studied on 73 patients and showed significant improvement of slip grade, lumbosacral angle, LL, and SS after partial reduction, fusion with instrumentation [16]. But reduction in adult HGS is generally more difficult and usually considered when the involved segment is unstable or there is a change in sagittal balance [17,24,25,39]. Regardless of patient age, HGS creates increased shear stress at the lumbosacral junction, with multiple studies reporting high rates of pseudarthrosis up to 40% and progression of the postoperative slip upto 26% without instrumentation even successful posterior arthrodesis after posterior in situ fusion [4,12,39,41]. This situation arises because a posterior fusion mass in HGS is exposed to high tensile forces. Despite higher pseudoarthrosis with in-situ fusion procedures, various authors have reported that the clinical and functional outcomes of in-situ fusion are comparable to circumferential fusion procedure with reduction [53]. Poussa reported on their long-term comparative study between reduction or in-situ fusion in severe adolescent spondylolisthesis and showed no significant difference for the clinical and functional outcomes between the two strategies and considered in-situ fusion when there is balanced pelvis (low pelvic tilt) or sagittally balanced spine and without neurological symptoms [54]. Molinari and colleagues [55] reported higher fusion rates following anterior support and arthrodesis when compared with posterior lateral fusion alone. Anterior interbody arthrodesis can be performed through separate anterior and posterior approach or through a posterior approach alone (PLIF or TLIF). If reduction is performed, circumferential (PLIF + PLF) fusion should be strongly considered to improve the overall biomechanics for fusion and greater stability. This procedure may be particularly relevant in patients with a high PI who have additional shear forces at the lumbosacral junction.
There are several advantages of reduction, fusion and instrumentation technique in HGS, among them, the reduction of the angle of slippage, which allows for neurological decompression, improvement of the lumbosacral sagittal orientation, which diminishes the shear forces across the fusion mass, improvement of cosmetic appearance, through the spontaneous correction of thoracic hypokyphosis and lumbar hyperlordosis as compared with the in-situ approaches [45,56]. In addition, instrumentation also helps in rapid mobilization as well as facilitate arthrodesis and maintain reduction as compared to in situ fusion [56]. In our study all radiographic parameters were significantly improved, including mean percentage of slip, slip angle, disc height and spino-pelvic parameters (SS, PT, PI and LL)] which were similar to other studies [11,16,31,40,45]. Smith et al [11] retrospectively reported 9 patients of HGS who were treated surgically by partial reduction, posterior interbody fusion and found significant improvement of the slip angle (41.20 to 210), lumbosacral kyphosis, sacral inclination and percentage of slippage post operatively at their 43 months follow-up. They concluded that partial reduction with fusion is an effective technique for the management of HGS. Another retrospective study also showed significant improvement of slip angle (620 to 280, P < 0.05) at 42.6 months follow-up without any neurologic complications or slip progression but developed 2 intraoperative dural tears [40]. A recent prospective study of 12 adult patients (mean age, 37 years) with HGS (III and IV) who underwent total or partial reduction by Spondylolisthesis Reduction Instrument (SRI) system with 3600 fusion and showed significant improvement of mean ODI scores from 59% to 12.4% (P < 0.05), percentage of slippage from 55% to 9.5% (P < 0.05), angle of slippage from 250 to 30 and sacral angle from 200 to 440 after surgery with an average follow-up of 29.1 months. No major complications such as deep infection, neurological damage or material breaks were observed [45] which were almost comparable to our study. In Shufflebarger and Geck [31] reported in 18 patients, using a one level reduction arthrodesis technique and showed significant improvement of slip percentage (77% to 13%) and slip angle (350 to 4.30) at final follow-up without any complications. Despite the advantages of reduction of HGS, higher risk of neurological injury especially the L5 and L4 roots (permanent or temporary) are associated with the reduction maneuver of the slip. However, cauda equina syndromes (CESs) have also been reported with reduction procedures [57,58]. In this study most of the patients, the percentage of slippage was reduced to less than 25% and was associated with 360º arthrodesis (PLA + PLIF), no cases of pseudoarthrosis were encountered and transient neuropraxia occurred only one patient (5%) though neurological deficits is common in HGS reduction maneuver. Probably some factors are responsible for reducing these risks, such as better exposure with laminectomy, visualization of the nerve roots during the reduction, 360º arthrodesis (PLA + PLIF) and the surgeon’s experience and ensuring that there is no distraction of the nerve elements.Limitations of this study was the small number of adult patients enrolled.
Conclusion
Partial posterior reduction, wide decompression and circumferential fusion (PLIF + PLF) with pedicle screw instrumentation is a viable option in adult High-Grade slip for relieving the symptoms, achieving stability and fusion.
References
- Kilian H. Schilderungen neuer beckenformen und ihres verhaltens im leben. Mannheim: verlag von bosserman; 1854.
- Herbiniaux G. Traite sur drivers accouchmements laborieux et sur lês polypes de la matrice. Brussels: JL Deboubers; 1782.
- Meyerding H. Spondylolisthesis: surgical treatment and results. Surg Gynecol Obstet 1984;54:371–377.
- Kasliwal MK, Smith JS, Kanter A, Chen CJ, Mummaneni PV, Hart RA, Shaffrey CL. Management of High-grade spondylolisthesis. Neurosurgery Clin North America. 2013;24(2):275–291.
- Transfeldt EE, Mehbod AA. Evidence-based medicine analysis of isthmic spondylolisthesis treatment including reduction versus fusion in situ for high-grade slips. Spine (Phila Pa 1976). 2007;32(19 Suppl):S126–S129..
- Passias PG, Poorman CE, Yang S, Boniello AJ, Jalai CM, Worley N, et al. Surgical treatment strategies for high-grade spondylolisthesis: a systematic review. Int J Spine Surg. 2015;9:50.
- Harris IE, Weinstein SL. Long-term follow-up of patients with grade-III and IV spondylolisthesis. Treatment with and without posterior fusion. J Bone Joint Surg Am. 1987;69(7):960–969.
- Goyal N, Wimberley DW, Hyatt A, Zeiller S, Vaccaro AR, Hilibrand AS, et al. Radiographic and clinical outcomes after instrumented reduction and transforaminal lumbar interbody fusion of mid and high-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2009;22(5):321–327.
- Gill GG, Manning JG, White HL. Surgical treatment of spondylolisthesis without spine fusion; excision of the loose lamina with decompression of the nerve roots. J Bone Joint Surg Am. 1955;37-A(3):493–520.
- Ruf M, Koch H, Melcher RP, Harms J. Anatomic reduction and monosegmental fusion in high-grade developmental spondylolisthesis. Spine. 2006;31(3):269–274.
- Smith JA, Deviren V, Berven S, Kleinstueck F, Bradford DS. Clinical outcome of trans-sacral interbody fusion after partial reduction for high-grade l5-s1 spondylolisthesis. Spine (Phila Pa 1976). 2001;26(20):2227–2234.
- Bohlman HH, Cook SS. One-stage decompression and posterolateral and interbody fusion for lumbosacral spondyloptosis through a posterior approach. Report of two cases. J Bone Joint Surg Am. 1982;64(3):415–418.
- Gaines RW. L5 vertebrectomy for the surgical treatment of spondyloptosis: thirty cases in 25 years. Spine (Phila Pa 1976). 2005;30(6 Suppl):S66–S70.
- Labelle H, Mac-Thiong J-M, Roussouly P. Spino-pelvic sagittal balance of spondylolisthesis: a review and classification. Eur Spine J. 2011;20 Suppl 5(Suppl 5):641–646.
- Labelle H, Roussouly P, Berthonnaud E, Transfeldt E, O’Brien M, Chopin D, et al. Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine (Phila Pa 1976). 2004;29(18):2049–2054.
- Labelle H, Roussouly P, Chopin D, Berthonnaud E, Hresko T, O’Brien M. Spino-pelvic alignment after surgical correction for developmental spondylolisthesis. Eur Spine J. 2008;17(9):1170–1176.
- DeWald CJ, Vartabedian JE, Rodts MF, Hammerberg KW. Evaluation and management of high-grade spondylolisthesis in adults. Spine (Phila Pa 1976). 2005;30(6 Suppl):S49–S59.
- Cheung EV, Herman MJ, Cavalier R, Pizzutillo PD. Spondylolysis and spondylolisthesis in children and adolescents: II. Surgical management. J Am Acad Orthop Surg. 2006;14(8):488–498.
- Freeman BL 3rd, Donati NL. Spinal arthrodesis for severe spondylolisthesis in children and adolescents. A long-term follow-up study. J Bone Joint Surg Am. 1989;71(4):594–598.
- Molinari RW, Bridwel KH, Lenke LG, Ungacta FF, Riew KD. Complications in the surgical treatment of pediatric high-grade, isthmic dysplastic spondylolisthesis. A comparison of three surgical approaches. Spine (Phila Pa 1976). 1999;24(16):1701–1711.
- Sailhan F, Gollogly S, Roussouly P. The radiographic results and neurologic complications of instrumented reduction and fusion of high-grade spondylolisthesis without decompression of the neural elements: a retrospective review of 44 patients. Spine (Phila Pa 1976). 2006;31(2):161–169..
- Seitsalo S, Osterman K, Hyvärinen H, Schlenzka D, Poussa M. Severe spondylolisthesis in children and adolescents. A long-term review of fusion in situ. J Bone Joint Surg Br. 1990;72(2):259–265.
- Lengert R, Charles YP, Walter A, Schuller S, Godet J, Steib J-P. Posterior surgery in high-grade spondylolisthesis. Orthop Traumatol Surg Res. 2014;100(5):481–484.
- Bradford DS. Treatment of severe spondylolisthesis. A combined approach for reduction and stabilization. Spine (Phila Pa 1976). Sep-Oct 1979;4(5):423–429.
- Poussa M, Schlenzka D, Seitsalo S, Ylikoski M, Hurri H, Osterman K. Surgical treatment of severe isthmic spondylolisthesis in adolescents. Reduction or fusion in situ. Spine (Phila Pa 1976). 1993;18(7):894–901.
- Harris IE, Weinstein SL. Long-term follow-up of patients with grade-III and IV spondylolisthesis. Treatment with and without posterior fusion. J Bone Joint Surg Am. 1987;69(7):960–969.
- Lonstein JE. Spondylolisthesis in children. Cause, natural history, and management. Spine (Phila Pa 1976). 1999;24(24):2640–2648.
- Bartolozzi P, Sandri A, Cassini M, Ricci M. One-stage posterior decompression-stabilization and trans-sacral interbody fusion after partial reduction for severe L5-S1 spondylolisthesis. Spine (Phila Pa 1976). 2003;28(11):1135–1141.
- Bridwell KH. Surgical treatment of high-grade spondylolisthesis. Neurosurg Clin N Am. 2006;17(3):331–338.
- Helenius I, Lamberg T, Osterman K, Schlenzka D, Yrjönen T, Tervahartiala P, et al. Posterolateral, anterior, or circumferential fusion in situ for high-grade spondylolisthesis in young patients: a long-term evaluation using the Scoliosis Research Society questionnaire. Spine (Phila Pa 1976). 2006;31(2):190–196.
- Shufflebarger HL, Geck MJ. High-grade isthmic dysplastic spondylolisthesis: monosegmental surgical treatment. Spine (Phila Pa 1976). 2005;30(6 Suppl):S42–S48.
- Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2–18.
- Pasha IF, Qureshi MA, Haider IZ, Malik AS, Qureshi MS, Tahir UB. Surgical treatment in lumbar spondylolisthesis: experience with 45 patients. J Ayub Med Coll Abbottabad. 2012;24(1):75–78.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940–2952.
- Canale ST, Beaty JH. Campbell’s operative orthopaedics, 12th edn. USA, Elsevier Mosby. 2013;1843.
- Vaz G, Roussouly P, Berthonnaud E, Dimnet J. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11(1):80–87.
- Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U. Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J. 2005;14(6):551–558.
- Yamamoto T, Ohta N, Minobe Y, Ohwada T. Posterior lumbar interbody fusion for degenerative spondylolisthesis. Neuro and spine surgery, Mumbai. 1996;589–590.
- Boxall D, Bradford DS, Winter RB, Moe JH. Management of severe spondylolisthesis in children and adolescents. J Bone Joint Surg Am. 1979;61(4):479–495.
- Boachie-Adjei O, Do T, Rawlins BA. Partial lumbosacral kyphosis reduction, decompression, and posterior lumbosacral transfixation in high-grade isthmic spondylolisthesis: clinical and radiographic results in six patients. Spine (Phila Pa 1976). 2002 Mar 15;27(6):E161–E168.
- Boos N, Marches D, Zuber K, Aebi M. Treatment of severe spondylolisthesis by reduction and pedicular fixation. A 4 years–6 years follow-up study. Spine (Phila Pa 1976). 1993;18(12):1655–1661.
- Edwards CC, Bradford DS. Instrumented reduction of spondylolisthesis. Spine (Phila Pa 1976). 1994;19(13):1535–1537.
- Roca J, Ubierna MT, Cáceres E, Iborra M. One-stage decompression and posterolateral and interbody fusion for severe spondylolisthesis. An analysis of 14 patients. Spine (Phila Pa 1976). 1999;24(7):709–714.
- Petraco DM, Spivak JM, Cappadona JG, Kummer FJ, Neuwirth MG. An anatomic evaluation of L5 nerve stretch in spondylolisthesis reduction. Spine (Phila Pa 1976). 1996;21(10):1133–1138.
- Abdala, Chiovato EH, Filgueira, Gonçalves E, Ferrer, Almeida Ld, et al.. High degree spondylolisthesis in adults: monosegmental reduction and fixation. Coluna/Columna. 2015;14(3);194–197.
- Newman PH. Degenerative spondylolisthesis. Orthop Clin North Am. 1975;6(1):197–198.
- Wynne-Davies R, Scott JH. Inheritance and spondylolisthesis: a radiographic family survey. J Bone Joint Surg Br. 1979;61-B(3):301–305.
- Legaye J, Duval-Beaupère G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J. 1998;7(2):99–103.
- Marty C, Boisaubert B, Descamps H, Montigny JP, Hecquet J, Legaye J, et al. The sagittal anatomy of the sacrum among young adults, infants, and spondylolisthesis patients. Eur Spine J. 2002;11(2):119–125.
- Hanson DS, Bridwell KH, Rhee JM, Lenke LG. Correlation of pelvic incidence with low- and high-grade isthmic spondylolisthesis. Spine (Phila Pa 1976). 2002;27(18):2026–2029.
- Hresko MT, Labelle H, Roussouly P, Berthonnaud E. Classification of high-grade spondylolistheses based on pelvic version and spine balance: possible rationale for reduction. Spine (Phila Pa 1976). 2007;32(20):2208–2013.
- O’Sullivan PB, Phyty GD, Twomey LT, Allison GT. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine (Phila Pa 1976). 1997;22(24):2959–2967.
- Muschik M, Zippel H, Perka C. Surgical management of severe spondylolisthesis in children and adolescents. Anterior fusion in situ versus anterior spondylodesis with posterior transpedicular instrumentation and reduction. Spine (Phila Pa 1976). 1997;22(17):2036–2042.
- Poussa M, Remes V, Lamberg T, Tervahartiala P, Schlenzka D, Yrjönen T. et al. Treatment of severe spondylolisthesis in adolescence with reduction or fusion in situ: long-term clinical, radiologic, and functional outcome. Spine (Phila Pa 1976). 2006;31(5):583–590.
- Molinari RW, Bridwel KH, Lenke LG, Baldus C. Anterior column support in surgery for high-grade, isthmic spondylolisthesis. Clin Orthop Relat Res. 2002;(394):109–120.
- Smith JA, Hu SS. Management of spondylolysis and spondylolisthesis in the pediatric and adolescent population. Orthop Clin North Am. 1999;30(3):487–499.
- Bradford DS, Boachie-Adjei O. Treatment of severe spondylolisthesis by anterior and posterior reduction and stabilization. A long-term follow-up study. J Bone Joint Surg Am. 1990;72(7):1060–1066.
- Ani N, Keppler L, Biscup RS, Steffee AD. Reduction of high-grade slips (grades III-V) with VSP instrumentation. Report of a series of 41 cases. Spine (Phila Pa 1976). 1991;16(6 Suppl):S302–S310.
Keywords
Adult spondylolisthesis; High-grade spondylolisthesis; Management; Surgery; Partial reduction
Cite this article
Ahsan MK, Yadav KK, Khan SI, Awwal MA, Joshi SR, Mahmud AA, et al. Surgical management of adult high-grade spondylolisthesis by partial reduction, decompression and instrumented circumferential fusion. Clin Surg J. 2021;2(2):1–9.
Copyright
© 2021 Ahsan MK. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).







