Management of Rectourethral Fistulas after Radical Prostatectomy: A University Hospital Experience
* Thomas Prudhomme;
Pierre Lunardi;
Matthieu Thoulouzan;
Guillaume Portier;
Nicolas Doumerc;
Xavier Gamé;
Michel Soulié;
Mathieu Roumiguié;
-
* Thomas Prudhomme: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Pierre Lunardi: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Matthieu Thoulouzan: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Guillaume Portier: Department of Digestive Surgery, Rangueil University Hospital, France.
-
Nicolas Doumerc: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Xavier Gamé: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Michel Soulié: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Mathieu Roumiguié: Department of Urology, Renal Transplantation and Andrology, Rangueil University Hospital, France.
-
Jan 15, 2021 |
-
Volume: 2 |
-
Issue: 1 |
-
Views: 4500 |
-
Downloads: 2245 |
Abstract
Introduction: Rectourethral Fistulas (RUF) after Radical Prostatectomy (RP) are an uncommon but serious complication, and there is no consensus on their management. In this study, we report the results of a standardized management procedure for RUF after RP.
Materials and methods: Twenty-one patients were treated for RUF after RP, 12 of which were performed by retropubic approach and 9 laparoscopically, from 2000 to 2019. First-line conservative treatment combining urinary diversion and GI rest through a temporary colostomy or prolonged residue-free diet were implemented. In the event of failure of this treatment at 3 months, surgery was performed. The follow-up was done at 3 months, 6 months and 12 months and then annually. Cases of recurrence were identified, as was urinary and fecal continence of patients after healing had occurred. The results were described in two groups: group 1 (success of the initial conservative treatment) and group 2 (failure of the initial conservative treatment).
Results: All the patients from group 1 had obtained sustained healing at the end of the conservative treatment. All of the following were found in this group: 1) no rectal wound identified intraoperatively; 2) normal Voiding Cystourethrography (VCUG) before postoperative removal of the urinary catheter; 3) appearance of symptoms beyond 10 postoperative days; and 4) no fecaluria or sepsis. In this group, the median restoration of continuity was 12 months. For all the patients in group 2, the radiographic assessment after 3 months of conservative treatment found no healing of the RUF. In this group, a rectal wound was identified intraoperatively in 50% of cases, 40% presented with clinical and radiological signs of RUF before the 8th postoperative day, with 50% severe symptoms (fecaluria, severe sepsis), and in 40% of cases the RP found a fistula > 1 cm. The median restoration of continuity was 14 months. In all patients taken together, the incontinence rate at one year was 66.7%. No fecal incontinence was described after the treatment.
Conclusion: RUF following radical prostatectomy is a complication that significantly alters the management of prostate cancer by delaying potentially indicated adjuvant treatment, as well as patient quality of life for a long period of time. Its management must be optimal in order to avoid a detrimental prolonged healing time by pinpointing the poor prognostic criteria intrinsic to the fistula, assisting with a rigorous clinical and paraclinical assessment, in order to determine the most suitable treatment regimen.
Introduction
Radical prostatectomy is the most common treatment for localized cancer of the prostate. In France in 2014, 17,400 radical prostatectomies were performed [1]. The surgical techniques of radical prostatectomy have gradually evolved over the past 20 years towards lower morbidity, with the advent of robotic surgery and the standardization of procedures [2]. However, in the case of extracapsular extension (stage ≥ pT3) [2], this surgical intervention remains at risk of postoperative complications, of which Rectourethral Fistula (RUF) is a rare but devastating component. Due to its exceptional incidence, it is difficult to establish a consensus from retrospective studies on the management of this complication, and the immediate and medium-term management frequently depends more on the practitioner and his or her experience with well-established decision-making algorithms [3–8]. This sometimes results to one or more unsuccessful repair attempts, eventually leading the practitioner to belatedly refer the patient to a specialized center and thus lengthening, and even jeopardizing, definitive healing. In this study, we evaluate the results of a standardized management procedure for RUF after RP. The aim was to investigate the poor prognostic criteria that would enable failure of first-line conservative treatment to be predicted.
Materials and Methods
Patients: This is a retrospective analysis of data from patients managed for rectourethral fistula after radical prostatectomy by retropubic approach or laparoscopically from January 2000 to December 2019. Patients referred for post-radiotherapy or brachytherapy RUF were excluded.
Diagnosis of RUF and initial management: Each RUF was confirmed through proctology exam under local and/or general anesthesia, as well as through endoscopic and radiographic exploration of the urethra (fibroscopy + Voiding Cystourethrography [VCUG]) and rectum (rectroscopy +/- rectography). When the RUF was not visualized with these methods, a pelvic MRI was done. All the patients had a first-line conservative approach with GI rest (either a GI diversion via left or transverse colostomy or a prolonged residue-free diet), as well as a urinary diversion via Foley catheter for a 6-week duration before clinical and radiological (VCUG) reassessment. Depending on the specialist practitioner, bilateral nephrostomy catheters were placed.
Surgical treatment of RUF: Beyond 3 months of unsuccessful conservative treatment, surgical treatment was implemented via approaches that included a similar proportion of abdominal, perineal (with interpositioning of a fat or collagen flap), trans-anal via direct approach or transsphincteric according to the York-Mason technique. The surgical techniques have been previously described [5,6,8–14] and details will not be given here. The choice of technique was based on the preoperative assessment data. Regardless of the intervention used, excision of the fistulous orifice was performed with closure of the urethra and rectum in two planes by PDS 4-0 separated sutures. An epiploplasty was done when local conditions permitted. The day prior to the intervention, mechanical washing of the rectum was done and an NPO status was maintained until the first postoperative day for patients with a colostomy, or a residue-free diet was followed for at least 4 weeks by patients without colostomy. The urinary catheter was kept in place for 6 weeks to 8 weeks.
Postoperative follow-up procedures: Patients were systematically seen as outpatients at 6 weeks, 3 months, 6 months and 1 year from treatment, each time with a physical examination, urethrovesical endoscopy and a VCUG. The urinary diversion was only removed when no fistula was found on the VCUG check-up. If the patient had a colostomy, restoration of continuity occurred at best 6 months after healing of the RUF.
Study endpoints and variables: The success of the procedure (closure of the fistula) was defined by the objective absence of rectourethral fistula visualized on a control VCUG 6 weeks to 8 weeks after the last treatment, together with the absence of clinical recurrence at least 6 months from the closure of the colostomy. Two groups were then formed based on the response to the initial conservative treatment: group 1 (success of the conservative treatment) and group 2 (failure of the conservative treatment). The following information was compiled from the patients’ medical file: age and BMI at the time of the Radical Prostatectomy (RP), history (surgery, prostatic or rectal, prostatitis, number of previous biopsies), preoperative clinical and paraclinical presentation, type of RP and intraoperative data (operative time, bleeding, rectal wound observed, nerve preservation, difficult apical dissection), pathological data (weight of the prostate, pTNM), duration of postoperative urinary catheterization, adjuvant treatment (RT, HT), time to diagnosis (time interval between the RP and the diagnosis of RUF), recurrence of fistula, number and type of surgical treatments. In addition, for each patient, a urinary continence assessment was performed (absence of leak or presence of urinary leak), along with a fecal continence assessment, which was estimated by proxy questionnaire using the Jorge and Wexner fecal incontinence score [15].
Results
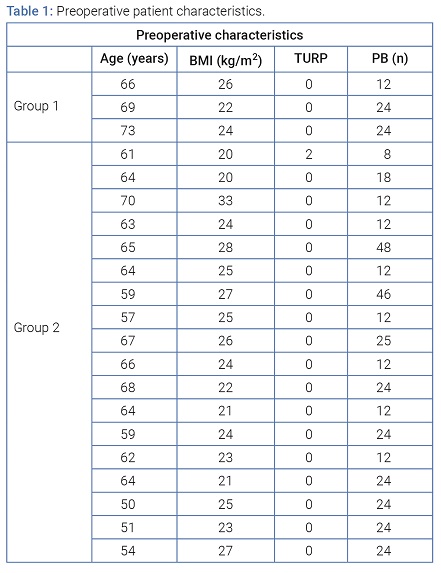
Preoperative patient characteristics: A total of 21 patients were treated for RUF after RP, 18 of whom were referred to our center. Twelve RPs were done by retropubic approach and 9 by laparoscopic approach. The conservative treatment was effective in 3 patients (Group 1), whereas a surgical intervention was needed in 18 patients (Group 2). The median age (IQR) was 64 years (59 years–66.5 years); the median (IQR) BMI was 24 kg/m2 (22-26 kg/m2). One patient had previously had two transurethral resections of the prostate. The median (IQR) number of preoperative transrectal prostatic biopsies was 24 (12–24), (Table 1).
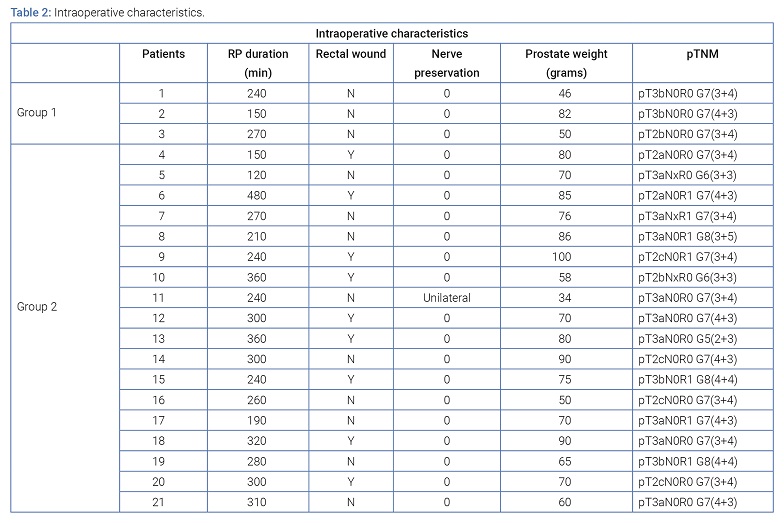
Intraoperative characteristics of radical prostatectomies: The median operative time (IQR) was 270 min (225–305), and the median operative blood loss was 350 ml (225–480). Unilateral nerve preservation was achieved in only one patient, while a difficult apical dissection during the RP occurred in seven patients. A rectal wound was identified intraoperatively and repaired in 9 of the 21 patients (42.9%). The medium prostatic volume was 70 g (59 g–83.5 g). The pathological analysis showed 8 (38.1%) pT2, 9 (42.9%) pT3a and 4 (19.0%) pT3b. A positive surgical margin was noted in 6 patients (28.6%); all margins were a millimeter in size and located at the apex of the prostate. The postoperative PSA was undetectable in all the patients, and in 2 of the 6 patients with a positive margin, adjuvant treatment with hormone therapy was started, (Table 2).
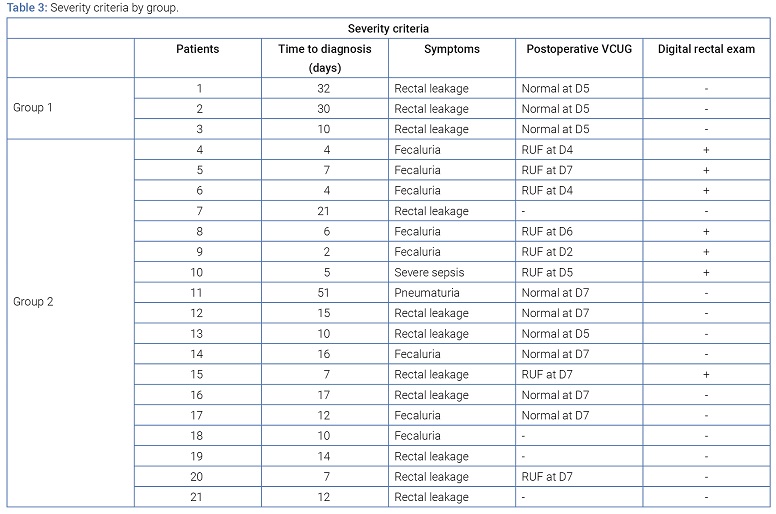
Severity criteria of RUF and details of the surgical treatment interventions of RUF: Nine patients (42.9%, Group 1: n = 3; Groupe 2: n = 6) had a normal VCUG before removal of the urinary catheter between the 5th and 7th post-RP day, and the RUF was then declared after the 10th postoperative day. The median time to the onset of symptoms was 10 days. The most common symptoms were leaking of urine through the anus as well as pneumaturia (57.1%), while 8 patients (38.1%) presented fecaluria. The fistula was opposite the urethrovesical anastomosis in 100% of cases. The median restoration of digestive continuity was 14 months (12 months–19.5 months) after the diagnosis of RUF. In group 1 (success in the initial conservative treatment), all 3 patients had a normal VCUG before removal of the urinary catheter between the 5th and 7th post-prostatectomy day, and the median time (IQR) from the onset of RUF symptoms to the diagnosis was 30 days (10 days–32 days). No initial fecaluria symptoms were found. In the three cases, no rectal wound was identified intraoperatively, and the RP did not find the RUF palpable after the onset of symptoms. Two patients did not have GI diversion, instead following a prolonged residue-free diet (RFD), and all had a urethral Foley catheter for 3 months. After 3 months of conservative treatment, the 3 patients achieved sustained healing, with median restoration of continuity at 12 months (7.5 months–19 months). In group 2 (failure of the initial conservative treatment), 8 of the 18 patients (44.4%) presented a clinical symptom of RUF before the 8th postoperative day. The median time to the diagnosis was 10 days (5.8 days–15.3 days). Eight patients (44.4%) developed fecaluria as the initial symptom, and one case (5.6%) of severe sepsis was noted, which required emergency surgical revision. In seven of them (39%), a fistula > 1 cm (i.e. admitting the pulp of the finger) was found during the RP. A rectal wound was identified in this group intraoperatively in 9 patients (50%). All the patients from this group had a diversion colostomy and bladder drainage via Foley catheter. Patients that initially had bilateral nephrostomy catheters were in this group. For all of these patients, the radiographic assessment after 3 months of conservative treatment found no healing of the RUF. The median time (IQR) for surgical management after RUF diagnosis was 6 months (5 months–8 months). The surgical approach used was abdominal in 3 patients, perineal with interpositioning of a fat or collagen flap in 3 patients, direct transanal in 5 patients or transsphincteric per the York-Mason technique in 7 patients. Five patients (27.8%) had RUF recurrence after a first surgical treatment, (Table 3 and 4).
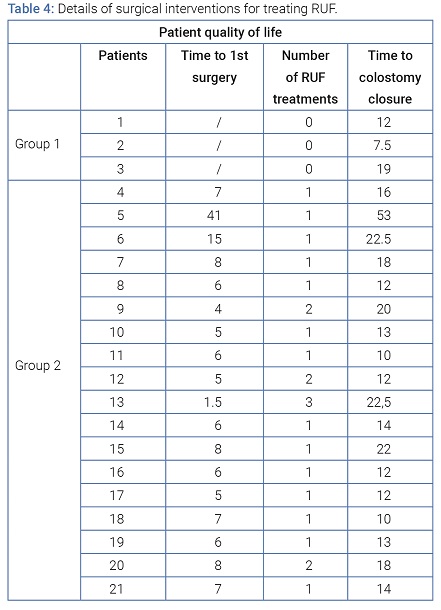
The first patient was operated at 15 months from the RUF diagnosis by transanal approach and had recurrence 12 months later. The treatment was then conservative with replacement of an indwelling urinary catheter for 3 months, which allowed definitive closure of the fistula. The second patient was operated at 4 months from the diagnosis by perineal approach with interpositioning of a collagen prosthesis, and had recurrence at 3 months. The second surgical intervention used the York-Mason posterior approach 6 months after the recurrence, which led to definitive healing. The third patient was operated at 5 months from the diagnosis via perineal approach with interpositioning of a pedicle fat flap, and had recurrence at 6 weeks (through necrosis of the fat interposition flap). The surgical revision was carried out by perineal approach with interpositioning of a pedicle gracilis muscle flap 7 months later. A second recurrence was observed at the 6-week follow-up; two nephrostomy catheters were then placed with an indwelling urinary catheter for an additional 6 weeks, which enabled drying of the RUF. The fourth patient was operated 6 months from the diagnosis via abdominal approach and showed recurrence on the first 6-week follow-up. The surgical revision took place 6 months later by transanal approach, and a second recurrence was observed. Three months later, surgical treatment via the York-Mason posterior approach enabled healing of the RUF.
Finally, the fifth patient was operated at 8 months from the diagnosis via perineal approach with interpositioning of a pedicle fat flap, and had recurrence at 6 weeks. The second surgery took place via York-Mason posterior approach 6 months after the recurrence, which resulted in definitive healing. At the end of a median follow-up (IQR) of 166.6 months (44 months–334 months), all of the patients had recovered from their RUF. The median restoration of continuity was 14 months (12 months–20 months). The York-Mason surgical technique resulted in healing of the RUFs each time. All the patients described stress urinary incontinence following removal of the urinary catheter after verification of sealing on the control VCUG, and this incontinence improved for 14 patients (66.7%) after one year of pelvic-perineal rehabilitation. One patient had an artificial periurethral sphincter placed via penoscrotal approach due to persistent urinary incontinence. After he had two surgical treatments for his RUF, only one patient presented with recurrent anastomotic stenosis despite multiple endoscopic urethrotomies and attempts at calibration of the urethra, which ultimately required a cystectomy with Bricker urinary diversion. All of the patients were interviewed with the Jorge and Wexner fecal incontinence questionnaire, and they all had a score below 2/20, attesting to absence of fecal incontinence after the management.
Discussion
Despite the rarity of occurrence of RUF, several studies have reported good results from different surgical techniques in the treatment of RUF following the management of prostate cancer (and particularly post-prostatectomy): trans-abdominal, perineal, trans-anal, transsphincteric, as well as combined techniques [3,5,7–13,16–20]. However, most of these series are heterogeneous with regard to the etiology of RUF, which can result from other therapeutic modalities of prostate cancer (radiotherapy, curietherapy, HIFU) [3,7,11,21–26] or even from pelvic trauma [27,28]. Indeed, the results of the initial therapeutic management were likely negatively influenced in case of radiation-injured tissue or severe local inflammation [6]. Moreover, these studies describe the feasibility and the results from different surgical techniques and approaches but very few identify the risk factors of failure of conservative treatment or the quality criteria of the initial management. However, this information is essential for improving patient quality of life and maximally reducing the risk of recurrence, which increases local fibrosis and makes subsequent repairs more difficult. The analysis of characteristics of the two groups provides valuable information on the potential risk factors of failure of conservative treatment. Thus, in group 1 no intraoperative wound had been noted, 100% of patients had a normal postoperative VCUG, a late onset of symptoms with a median time to diagnosis of 30 days, no fecaluria type symptoms and/or sepsis, and the RP found no palpable fistula, indicating a small caliber fistula diameter. In contrast, in group 2 only 33% had a normal postoperative VCUG, the symptoms occurred early, with a median time to diagnosis of 10 days, 50% of patients had fecaluria or sepsis, and in 40% of them the RP found a fistula > 1cm, indicating rather a large caliber RUF. These observations tend to regard two types of patients during the initial diagnosis, those having a good prognosis with a small caliber RUF and the others having a poorer prognosis with a large caliber RUF. Few preoperative factors appear to be correlated with iatrogenic RUF risk except for a large number of preoperative biopsies (24 in this series) and a high prostatic weight (70 grams on average in our series), which can be assumed to increase the surgical difficulties, including rectal adhesions at the posterior plane [3,6]. The pathological data are also consistent with this since 13 out of 21 patients (62%) had a locally advanced stage (≥ pT3a), and 6 cases with positive margins (28.6%) all occurred in patients with difficult apical dissection. In our series, a rectal wound was identified and repaired in 9 of the 21 patients (42.9%). This is probably the most important iatrogenic risk factor of RUF [29]. These results are consistent with those previously published, with the incidence of rectal lesions identified intraoperatively varying from 0.5% to 9% according to the series [6,30–32]. In our series, all the patients initially received conservative treatment combining urinary diversion and GI diversion through colostomy or residue-free diet. The rate of spontaneous closure after colostomy was only 4.8% (1 patient out of 21), whereas among the patients with a good prognosis, 2 out of 3 (66%) obtained first intention healing with a residue-free diet. It therefore seems that in cases with a good prognosis, urinary diversion combined with a residue-free diet has a chance of success. Rates of spontaneous closure for conservative treatment without initial colostomy have been described that vary from 14% to 54% according to the series. Reports of fecaluria and sepsis are also defined as poor prognostic factors, as their presence contraindicates surgical intervention for FUR without prior GI diversion by colostomy due to significant local inflammation [6,13,33–36]. Taken together with the clinical characteristics, an initial assessment, including a proctology exam under local and/or general anesthesia, endoscopic exploration and an x-ray of the urethra and rectum, seems essential for specifying the location and diameter of the fistula. As necessary, these will guide the surgical team in choosing the approach and the surgical technique. Although no studies for now have shown the superiority of one technique over another [37], it seems that the best approach the one that guarantees the best exposure for dissecting, excising the fistula tract and making an optimal suture. When feasible, performing an epiploplasty is of critical importance. However, there is consensus concerning the best time period for RUF closure after colostomy, which is after 3 months since control of local inflammation is crucial. This time interval was respected in our series of cases, with satisfactory results since only 5 patients out of 18 (27.8%) had to resort to more than one surgical treatment. Rapid resolution of symptoms through management optimization impacts the patient’s quality of life since the mean time to restoration of digestive continuity was 12 months in group 1 (7.5 months–19 months) vs. 14 months in group 2 (12 months–21 months). However, all the observations drawn from our experience and the data in the literature enabled us to construct a decision tree as presented in (Figure 1).
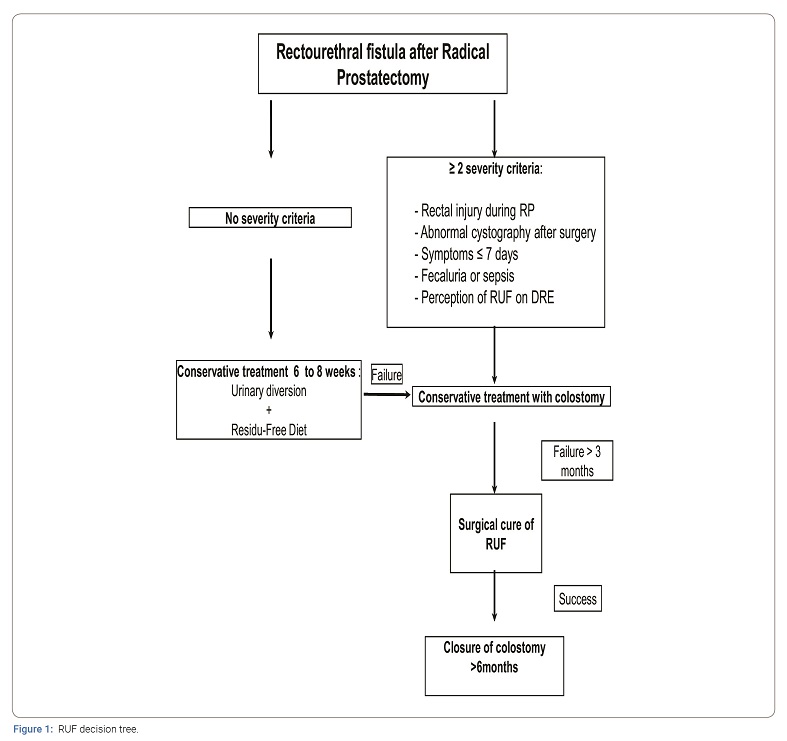
The absence at diagnosis of poor prognostic criteria indicating a significant RUF, including 1) an intraoperative rectal wound with abnormal post-RP VCUG; 2) the early (< 10 days) and sudden onset (fecaluria, severe sepsis) of symptoms; and 3) an RP that finds a large fistula > 1cm, should initially prompt consideration of conservative treatment combining urinary diversion via urinary bladder catheter or suprapubic catheter and GI rest with a residue-free diet for at least 6 weeks, with a control VCUG at the end of this period. In contrast, or in case of failure of conservative treatment, the presence of at least two of these poor prognostic criteria should initially prompt the implementation of a colostomy associated with urinary diversion for at least 3 months, which is the minimum interval before considering surgical treatment of the RUF. Restoration of GI continuity should be best achieved after 6 months without signs of recurrence, especially in patients at risk of local relapse of prostate cancer (positive margins, stage ≥ pT3) and therefore likely to be candidates for adjuvant treatment (RT, HT), which can jeopardize healing of the fistula tract.
Conclusion
RUF following radical prostatectomy is a complication that significantly alters the management of prostate cancer by delaying potentially indicated adjuvant treatment, as well as patient quality of life for a long period of time. Its management must be optimal in order to avoid a detrimental prolonged healing time by pinpointing the poor prognostic criteria intrinsic to the fistula, assisting with a rigorous clinical and paraclinical assessment, in order to determine the most suitable treatment regimen.
References
- Soulie M, Salomon L. [The issue of prostate cancer surgery]. Prog Urol. 2015;25(15):916–917.
- Rozet F, Hennequin C, Beauval JB, Beuzeboc P, Cormier L, Fromont-Hankard G, et al. [French ccAFU guidelines - Update 2018-2020: Prostate cancer]. Prog Urol. 2018;28(Suppl 1):R81–R132.
- Rouanne M, Vaessen C, Bitker MO, Chartier-Kastler E, Roupret M. Outcome of a modified York Mason technique in men with iatrogenic urethrorectal fistula after radical prostatectomy. Dis Colon Rectum. 2011;54(8):1008–1013.
- Hanus T. Rectourethral fistulas. Int Braz J Urol. 2002;28(4):338–345.
- Munoz M, Nelson H, Harrington J, Tsiotos G, Devine R, Engen D. Management of acquired rectourinary fistulas: outcome according to cause. Dis Colon Rectum. 1998;41(10):1230–1238.
- Thomas C, Jones J, Jager W, Hampel C, Thuroff JW, Gillitzer R. Incidence, clinical symptoms and management of rectourethral fistulas after radical prostatectomy. J Urol. 2010;183(2):608–612.
- van der Doelen MJ, Fransen van de Putte EE, Horenblas S, Heesakkers JPFA. Results of the York Mason procedure with and without concomitant graciloplasty to treat iatrogenic rectourethral fistulas. Eur Urol Focus. 2020;6(4):762–769.
- Bergerat S, Rozet F, Barret E, da Costa JB, Castro A, Dell’oglio P, et al. Modified York Mason technique for repair of iatrogenic recto-urinary fistula: 20 years of the Montsouris experience. World J Urol. 2018;36(6):947–954.
- Zmora O, Potenti FM, Wexner SD, Pikarsky AJ, Efron JE, Nogueras JJ, et al. Gracilis muscle transposition for iatrogenic rectourethral fistula. Ann Surg. 2003;237(4):483–487.
- Crippa A, Dall’oglio MF, Nesrallah LJ, Hasegawa E, Antunes AA, Srougi M. The York-Mason technique for recto-urethral fistulas. Clinics (Sao Paulo). 2007;62(6):699–704.
- Fengler SA, Abcarian H. The York Mason approach to repair of iatrogenic rectourinary fistulae. Am J Surg. 1997;173(3):213–217.
- Stephenson RA, Middleton RG. Repair of rectourinary fistulas using a posterior sagittal transanal transrectal (modified York-Mason) approach: an update. J Urol. 1996;155(6):1989–1991.
- Renschler TD, Middleton RG. 30 years of experience with York-Mason repair of recto-urinary fistulas. J Urol. 2003;170(4 Pt 1):1222–1225; discussion 1225.
- Kilpatrick FR, Mason AY. Post-operative recto-prostatic fistula. Br J Urol. 1969;41(6):649–654.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Boushey RP, McLeod RS, Cohen Z. Surgical management of acquired rectourethral fistula, emphasizing the posterior approach. Can J Surg. 1998;41(3):241–244.
- Quazza JE, Firmin F, Cossa JP. [Recto-urethral fistula following prostatectomy: surgical repair using a combined perineal and laparoscopic approach. Procedure and results of two cases]. Prog Urol. 2009;19(6):434–437.
- Lefevre JH, Bretagnol F, Maggiori L, Alves A, Ferron M, Panis Y. Operative results and quality of life after gracilis muscle transposition for recurrent rectovaginal fistula. Dis Colon Rectum. 2009;52(7):1290–1295.
- Hanna JM, Turley R, Castleberry A, Hopkins T, Peterson AC, Mantyh C, et al. Surgical management of complex rectourethral fistulas in irradiated and nonirradiated patients. Dis Colon Rectum. 2014;57(9):1105–1112.
- Hadley DA, Southwick A, Middleton RG. York-Mason procedure for repair of recto-urinary fistulae: a 40-year experience. BJU Int. 2012;109(7):1095–1098.
- Marguet C, Raj GV, Brashears JH, Anscher MS, Ludwig K, Mouraviev V, et al. Rectourethral fistula after combination radiotherapy for prostate cancer. Urology. 2007;69(5):898–901.
- Shakespeare D, Mitchell DM, Carey BM, Finan P, Henry AM, Ash D, et al. Recto-urethral fistula following brachytherapy for localized prostate cancer. Colorectal Dis. 2007;9(4):328–331.
- Elliott SP, McAninch JW, Chi T, Doyle SM, Master VA. Management of severe urethral complications of prostate cancer therapy. J Urol. 2006;176(6 Pt 1):2508–2513.
- Lane BR, Stein DE, Remzi FH, Strong SA, Fazio VW, Angermeier KW. Management of radiotherapy induced rectourethral fistula. J Urol. 2006;175(4):1382–1387; discussion 1387–1388.
- Chrouser KL, Leibovich BC, Sweat SD, Larson DW, Davis BJ, Tran NV, et al. Urinary fistulas following external radiation or permanent brachytherapy for the treatment of prostate cancer. J Urol. 2005;173(6):1953–1957.
- Linder BJ, Umbreit EC, Larson D, Dozois EJ, Thapa P, Elliott DS. Effect of prior radiotherapy and ablative therapy on surgical outcomes for the treatment of rectourethral fistulas. J Urol. 2013;190(4):1287–1291.
- Youssef AH, Fath-Alla M, El-Kassaby AW. Perineal subcutaneous dartos pedicled flap as a new technique for repairing urethrorectal fistula. J Urol. 1999;161(5):1498–1500.
- Pieretti RV, Pieretti-Vanmarcke RV. Combined abdominal and posterior sagittal transrectal approach for the repair of rectourinary fistula resulting from a shotgun wound. Urology. 1995;46(2):254–256.
- Mandel P, Linnemannstons A, Chun F, Schlomm T, Pompe R, Budaus L, et al. Incidence, risk factors, management, and complications of rectal injuries during radical prostatectomy. Eur Urol Focus. 2018;4(4):554–557.
- Harpster LE, Rommel FM, Sieber PR, Breslin JA, Agusta VE, Huffnagle HW, et al. The incidence and management of rectal injury associated with radical prostatectomy in a community based urology practice. J Urol. 1995;154(4):1435–1438.
- McLaren RH, Barrett DM, Zincke H. Rectal injury occurring at radical retropubic prostatectomy for prostate cancer: etiology and treatment. Urology. 1993;42(4):401–405.
- Gillitzer R, Melchior SW, Hampel C, Wiesner C, Fichtner J, ThUroff JW. Specific complications of radical perineal prostatectomy: a single institution study of more than 600 cases. J Urol. 2004;172(1):124–128.
- Noldus J, Fernandez S, Huland H. Rectourinary fistula repair using the Latzko technique. J Urol. 1999;161(5):1518–1520.
- Nyam DC, Pemberton JH. Management of iatrogenic rectourethral fistula. Dis Colon Rectum. 1999;42(8):994–997; discussion 997–999.
- al-Ali M, Kashmoula D, Saoud IJ. Experience with 30 posttraumatic rectourethral fistulas: presentation of posterior transsphincteric anterior rectal wall advancement. J Urol. 1997;158(2):421–424.
- Gupta G, Kumar S, Kekre NS, Gopalakrishnan G. Surgical management of rectourethral fistula. Urology. 2008;71(2):267–271.
- Hechenbleikner EM, Buckley JC, Wick EC. Acquired rectourethral fistulas in adults: a systematic review of surgical repair techniques and outcomes. Dis Colon Rectum. 2013;56(3):374–383.
Keywords
Fistula; Rectal injury; Iatrogenic; Complications; Radical prostatectomy; York mason; Surgery
Cite this article
Prudhomme T, Lunardi P, Thoulouzan M, Portier G, Doumerc N, Gamé X, Soulié M, Roumiguié M. Management of rectourethral fistulas after radical prostatectomy: a university hospital experience. Clin Surg J. 2021;2(1):1–8.
Copyright
© 2021 Thomas Prudhomme. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).





