Breast Tumor Volume to Breast Volume Ratio Analysis to Predict Breast Cancer Axillary Node Positivity Rates
Rayen J;
* Poole GH;
Hill AG;
-
Rayen J: Department of General Surgery, Middlemore Hospital, Otahuhu, Auckland, New Zealand.
-
* Poole GH: Department of General Surgery, Middlemore Hospital, Otahuhu, Auckland, New Zealand.
-
Hill AG: Department of General Surgery, Middlemore Hospital, Otahuhu, Auckland, New Zealand.
-
Jun 08, 2020 |
-
Volume: 1 |
-
Issue: 3 |
-
Views: 3911 |
-
Downloads: 2131 |
Abstract
Background: Prognostic scoring systems such as TNM [1] and NPI (Nottingham prognostic index) [2] use maximal tumour diameter for staging. Breast cancer is three-dimensional therefore tumour volume and weight could be more accurate biological predictors. Nodal spread may also be different for tumors occupying a greater or lesser proportion of the breast. This study aims to measure breast cancer weights as a proportion of the mastectomy specimen weight and to compare node positivity rates in high and low proportion specimens.
Methods: Size, grade, node positivity, lymphovascular invasion, receptors, quadrant and tumour type were recorded for 382 mastectomy specimens. The weights of carcinomas were estimated as a proportion of the entire mastectomy weight. Tumour volume and weight was estimated using the ellipsoid model (V=(abc)π/6). The cohort was then divided at the medians into “low” and “high” tumour proportionality and “large” and “small” tumours. Tumour weight and grade were then standardised for node positivity analysis.
Results: The overall node positivity (ITC, microinvasion and macroinvasion) rate was 194/382 (51%). In tumours with a low proportionality 37.6% (72/191) were node positive and in the high proportionality group 63.8% (122/191) were node positive (p =< 0.00001). Grade 1 tumours were node positive in 16/44 and grade 3 were positive in 84/155 (p = 0.0002). Small volume tumours were positive in 70/191 and large volume were positive in 124/191.The smallest tumour to breast weight ratio was 0.00001 and the largest was 0.709.
Conclusion: These data suggest that surgeons should calculate the three-dimensional proportion of the breast that is occupied by cancer, particularly when the patient is on the cusp of sentinel node biopsy versus axillary dissection.
Introduction
Breast cancer is the most common cancer among New Zealand women and follows lung cancer as the leading cause of cancer death of New Zealand females [3]. The overall age-standardised mortality rate is higher in New Zealand Maori compared to non-Maori women [4].
Breast cancersize strongly correlates axillary node positivity and therefore prognosis [5]. The MSKCC (Memorial Sloane Kettering Cancer Center) nomogram [6] for sentinel node metastases includes tumour size as a predictor, with a larger tumour size associated with a higher probability of node positivity and therefore poorer prognosis. Major scoring systems in breast cancer such as TNM [1] and NPI (Nottingham prognostic index) [2], use maximal diameter rather than volume to predict axillary node positivity. However, volume of cancer is thought to be a more biologically appropriate representation of tumour burden [7], therefore may be a more precise tool for determining the probability of axillary node positivity in breast cancer. The accuracy in predicting node positivity preoperatively is vital, because as the suspicion of node positivity increases, the operative management at the axilla is more aggressive, that is, axillary dissection over sentinel node biopsy.
Tumour spreads to lymph nodes via lymphatics, and since lymphatics and lymph nodes are known to have biological activity against carcinoma [8], it is possible that a larger breast may protect from axillary node spread. Therefore, since the mean breast volume varies between ethnicities [9], there may be ethnic differences in the probability of axillary node metastases. To date, there has been a paucity of research published on the effect of breast cancer volume to breast volume ratio on axillary node metastases [10,11], and none looking specifically at outcomes in the Maori population of New Zealand.
The aim of this study was to firstly determine how effective tumour to breast volume is as a predictor of axillary node positivity. We then explored whether the proportion of breast cancer to breast volume could be used as a predictor of axillary node positivity. Finally, we analysed ethnic variations in breast volume, breast tumour to volume ratio, and axillary node positivity.
Methods
Study population: Records from 500 consecutive patients who had mastectomies between 2013 to 2017 were reviewed. Inclusions were women who had mastectomies for invasive breast carcinoma. Exclusions included women who had undergone previous breast surgery or had undergone neoadjuvant chemotherapy. Women that had Paget’s disease or had missing tumour dimensions or breast volume were also excluded.
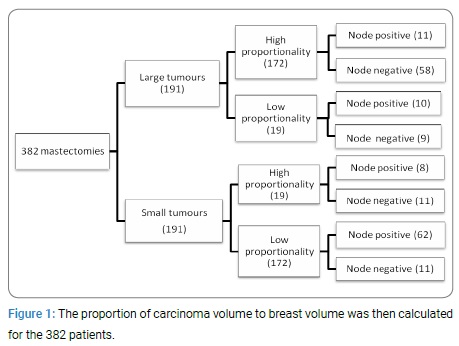
Data collection and Statistical analyses: Variables included in the MSKCC nomogram for sentinel node metastases such as age at diagnosis, tumour dimensions, cancer type, quadrant, lymphovascular invasion, grade, multifocality, receptor status, as well as ethnicity, smoking and node positivity were documented from clinical electronic records. Node positivity included micrometastases, macrometastases as well as isolated tumour cells to capture any evidence of tumour activity. In cases of satellite lesions in the breast, the volume of the largest lesion with invasive carcinoma was used. Volume of breast was derived by weight, with the assumption that one kilogram of breast tissue equated to one litre. Volume of cancer was approximated using the ellipsoid formula (V=(abc)π/6), where a, b and c represent the height, width and depth of the tumour. The ellipsoid formula has previously been used for estimating tumour volume and has been validated using a water displacement method [10]. The proportion of carcinoma volume to breast volume was then calculated for the 382 patients. The cohort was then divided at the medians into “low” and “high” tumour proportionality and “large” and “small” tumours (Figure 1). Logistical regression was used to determine association with node positivity and multivariate analysis was used to standardise for other variables.
Results
After the appropriate exclusions were made, 382 consecutive mastectomy specimens with invasive carcinoma were examined. The median age of diagnosis in the study population was 58 (range 58.6 to 88). The mean breast volume was 1043g (range 94g to 3000g) and the mean tumour volume was 16.7 cm3 (range 0.006 cm3 to 490.1 cm3). The ratio of tumour to breast volume ranged from 0.001% to 70.9% with a mean of 1.8% and median of 6%.
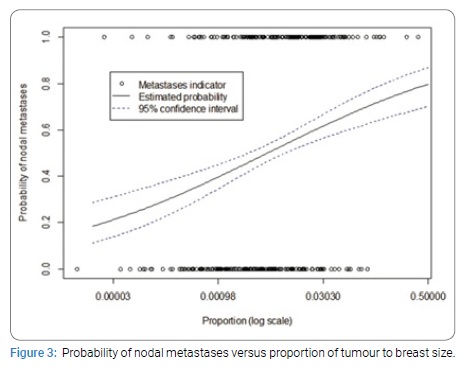
The overall axillary node positivity rate was 194/382 (51%). 36.6% (70/191) of small volume tumours and 64.9% (124/191) of large volume were node positive (p-value < 0.00000003). In tumours with a low proportionality, 37.6% (72/191) were node positive and 63.8% (122/191) of the high proportionality group were node positive (p =< 0.00001). Grade was associated with node positivity as expected. Grade 1 tumours were node positive in 16/44 (35.6%) and grade 3 were positive in 84/155 (54.2%) (p = 0.0002). In both large and small volume tumours there was a difference in high and low proportionality; p = 0.0003 (Table 1).
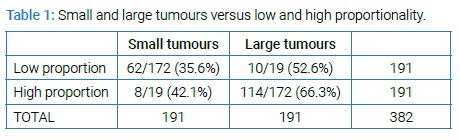
Multivariate analysis was then conducted, with adjustments made for age, grade, ethnicity, subtype, receptors and multifocality. A graphical investigation of the distribution of the tumour to breast volume ratio and the tumour volume led them to be transformed logarithmically and for ease of interpretation, the base 2 logarithm was selected. Doubling of tumour volume was associated with node positivity (OR 1.33, 95% CI 1.21–1.47, p-value = 6e-09) (Figure 2).
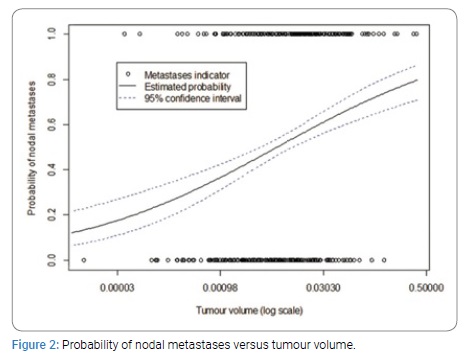
Doubling of tumour to breast volume ratio was associated with node positivity (OR 1.30, 95% CI 1.17–1.45, p-value = 1e-06) (Figure 3).
When tumour volume was standardised in this analysis however, this relationship became non-significant (OR 0.96, 95% CI 0.72–1.29, p-value = 0.80) Of the 382 women included in the study, 15.5% were NZ Maori (Table 2).
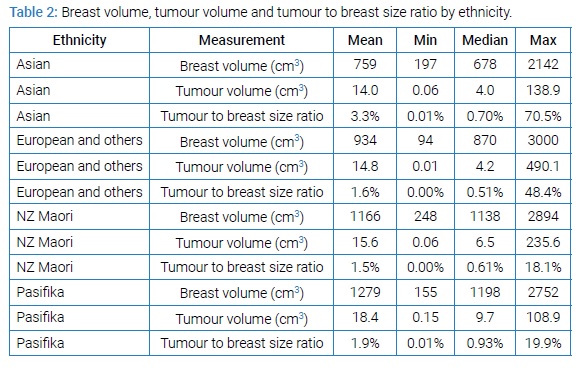
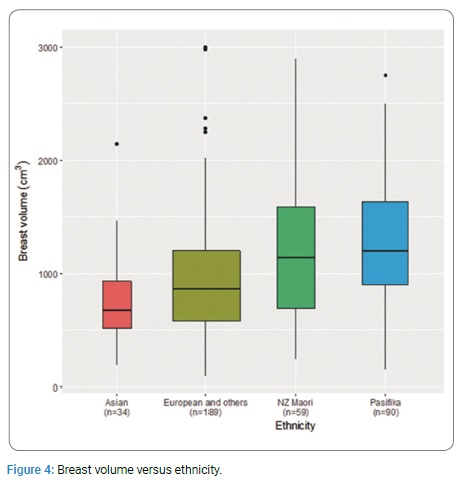
NZ Maori had a mean mastectomy weight 29% greater than NZ European (1138g vs. 887g). NZ Maori also had a larger tumour to breast volume ratio compared with NZ European (median 0.61% vs. 0.53%) (Figure 4).
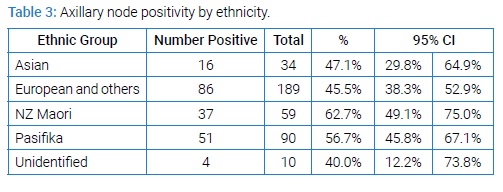
NZ Maori women had higher rates of nodal positivity compared to NZ European (62.7% vs. 45.5% p = 0.074) despite having proportionally less grade three tumours than NZ European (35.6% vs. 40.1%) (Table 3).
Multivariate analysis revealed that ratio of tumour to breast volume in NZ Maori compared to European was associated with higher rates of node positivity but this relationship with not significant (OR 1.91, 95% CI 1.01–3.63, p-value = 0.94).
Discussion
Axillary lymph node status is the most important prognostic factor of overall survival in breast cancer [12]. This study demonstrates that breast tumour volume is a strong independent predictor of axillary node positivity. Our analyses also show that a high tumour to breast volume ratio is associated with high rates of axillary node positivity. In essence, the further the tumour has to travel to the axilla, the lower the chance of node positivity whether large or small tumour, however this relationship loses significance when multivariate analysis is applied, due to the strong correlation between tumour volume and tumour to breast volume ratio. NZ Maori had a higher mean breast volume compared to European but a larger tumour to breast volume ratio, with increased rates of axillary node positivity.
To date there have been very few studies looking at the proportion of breast cancer to breast volume and its relation to axillary lymph node metastases. Wen J, et al. study [10] provided strong evidence to suggest that tumour-to-breast volume ratio is an independent prognostic factor of cancer-specific survival and positively associated with the status of axillary lymph node metastases. The calculations were slightly different in that the cohort was categorized into low and high tumour-to-breast volume ratios based on an optimal cutoff determined to be at 1.70%, in contrast to our study where the division was at the median proportion. Martić, et al. [11] was the first group to analyse tumour to breast volume ratio, proving it to be an independent predictive factor for axillary lymph node metastases in T1c ductal invasive breast cancer. Their methodology was markedly different from the present study as well as that of Wen J, et al. study [10]. In the first instance, the study was narrowed to include only T1c ductal invasive breast cancer. Breast volume was measured preoperatively using a device measuring according to a device based on Archimedes’ principle.
Although there is well-established relationship between tumour size and lymph node status in breast cancer [13], the main measurement used for tumour size has been tumour diameter. To our knowledge, our observation that tumour volume is strongly associated with axillary node positivity has only previously been studied by Wen J, et al. [10] who support this finding. Since volume of tumour is a more direct indicator of tumour burden, it may be more helpful to surgeons to think about tumour size in these terms rather than diameter, when considering the impact of size on axillary metastases.
Identifying predictors of axillary node metastases is important in order to reduce the false negative rate of sentinel node biopsies and axillary node dissections, as well as reduce the need for extensive surgery at the axilla. The have been a vast array of radiographical methods described in the literature [14–16] for preoperative measurement of breast and tumour volume. Calculating tumour to breast volume ratio preoperatively using some of these techniques, may provide surgeons with more information in deciding the most appropriate management of the axilla.
Prior studies have shown that the NZ Maori and Pacific Island population of New Zealand have larger and higher grade breast carcinoma with more involved lymph nodes [9]. This has been attributed to variation in biological characteristics. Our study reinforces the fact that NZ Maori have higher rates of axillary node positivity and larger tumours compared to NZ European. There are also clear ethnic differences in the proportion of breast cancer to breast volume illustrated in this study. This may have implications in terms of closer monitoring and more aggressive management of breast cancer in certain ethnic populations.
Our study was limited by the small number of participants, which affected our ability to achieve statistical significance. Menopausal status was not accounted for in our analysis, and therefore the effect of estrogen on breast and tumour volume was not considered. The hypothesis behind the study assumes that a larger breast equates to longer lymphatics however this has not yet been proven and wet lab studies on lymphatics need to be done to provide evidence for this.
Conclusion
The present study demonstrates the power of using breast cancer volume to predict metastatic spread to the axilla. It also adds to the current evidence for using the proportion of tumour to breast volume to predict axillary node metastases. These data suggest that surgeons should calculate the three-dimensional proportion of the breast that is occupied by cancer, particularly when the patient is on the cusp of sentinel node versus axillary dissection. Further investigation should be undertaken to explore reasons for Maori having a higher risk of nodal spread compared to non-Maori, in order to reduce disparities in breast cancer outcomes.
References
- Brierly J GM, Wittekind C. TNM classification of malignant tumours. Oxford: Union for international cancer control. 2017.
- Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, et al. A prognostic index in primary breast cancer. British journal of cancer. 1982;45(3):361–366.
- Health Mo. New Registrations and Deaths 2013 Wellington: Ministry of Health, 2016.
- Health Mo. Cancer in new zealand: trends and projections. Wellington: ministry of health, 2002.
- Carter CL, Allen C, Henson DE. Relation of tumour size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989:63:181–187.
- Bevilacqua JL KM, Fey JV, Cody HS, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. Journal of clinical oncology. 2007;25(24):3670–3679.
- Tsai CH LC, Hsieh CC, Hsu WH, Wang HW, Wang LS. Tumor volume is a better prognostic factor than greatest tumor diameter in stage Ia non-small cell lung cancer. Thorac Cardiovasc Surg. 2006;54:537–543.
- Ji R-C. Lymph nodes and cancer metastasis: new perspectives on the role of intranodal lymphatic sinuses. Int J Mol Sci. 2017;18(1):51.
- Weston MK, Moss DP, Stewart J, Hill AG. Differences in breast cancer biological characteristics between ethnic groups in New Zealand. Breast Cancer Research and Treatment. 2008;111(3):555–558.
- Wen J YF, Huang X, Li S, Yang L, Xiao X, Xie X. The tumor-to-breast volume ratio (TBR) predicts cancer-specific survival in breast cancer patients who underwent modified radical mastectomy. Tumour Biol. 2016;37(6):7493–7500.
- Stanec KMZVFRSLČT-LZ. Tumor and breast volume ratio as a predictive factor for axillary lymph node metastases in t1c ductal invasive breast cancer: prospective observational clinico-pathological study. Japanese Journal of Clinical Oncology. 2011;41(12):1322–1326.
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. Cancer. 1983;52:1551–1557.
- Elahe Orang ETM, Aboulfazl Afsharfard. Predictive role of tumor size in breast cancer with axillary lymph node involvement - can size of primary tumor be used to omit an unnecessary axillary lymph node dissection? Asian Pacific journal of cancer prevention. 2013;14(2):717–722.
- Rebecca Leddy, Abid Irshad, Allie Metcalfe, Pramod Mabalam, Ahad Abid, Susan Ackerman, et al. Comparative accuracy of preoperative tumor size assessment on mammography, sonography, and MRI: Is the accuracy affected by breast density or cancer subtype?. Journal of clinical ultrasound 2016;44(1):17–25.
- Ines V Gruber, Miriam Rueckert, Karl O Kagan, Annette Staebler, Katja C Siegmann, Andreas Hartkopf, et al. Measurement of tumour size with mammography, sonography and magnetic resonance imaging as compared to histological tumour size in primary breast cancer. BMC Cancer. 2013;13:328.
- Lagendijk M, Vos EL, Ramlakhan KP, Verhoef C, Koning AHJ, van Lankeren W, et al. Breast and tumour volume measurements in breast cancer patients using 3-d automated breast volume scanner images. World Journal of Surgery. 2018;42(7):2087–2093.
Keywords
Carcinoma; Tumor; Breast Cancer
Cite this article
Rayen J, Poole GH, Hill AG. Breast tumor volume to breast volume ratio analysis to predict breast cancer axillary node positivity rates. Clin Surg J. 2020;1(3):1–5.
Copyright
© 2020 Poole GH. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-4.0).







